Pathways to Better Health
From structural biology to drug discovery
Tuberculosis (TB) is one of the top 10 causes of death worldwide, claiming the lives of 1.6 million people in 2017 alone. Life scientists at Nankai, led by Rao Zihe, a member of the Chinese Academy of Sciences (CAS), are investigating the molecular mechanisms behind the disease. “To end the TB epidemic and invent new, effective drugs, we need to enhance basic research,” says Rao. “New drug discovery cannot be separated from research on target protein structure and functions.”
The unusual structure and chemical composition of Mycobacterium tuberculosis (Mtb), the pathogenic bacteria that causes TB, hinders drug entry and limits antibiotic effectiveness. Rao’s team is focussing efforts on target proteins for Mtb — in particular, a group of proteins called Mycobacterial membrane protein Large, or MmpL for short. This group of proteins is responsible for transporting lipids in the Mtb cell walls, and plays a major role in the bacterium’s efforts to infect and remain in the host.
Using X-ray crystallography, Rao’s team determined the high-resolution crystal structures of MmpL3, as well as complexes of MmpL3, and four TB drug candidates. They discovered how the candidate molecules bind with MmpL3, and found a transmembrane region where the inhibitor molecule blocks the energy supply channel of MmpL3 protein, causing its dysfunction.
This finding could help researchers develop MmpL3 inhibitors as new drugs for TB, leprosy, and other diseases.
As MmpL proteins exist in many pathogens and cause antibiotic resistance, structural analysis of these proteins by Rao’s team has also paved the way for finding solutions to antibiotic resistance.
Energy metabolism in Mycobacteria, in particular, the process in which ATP is formed, called oxidative phosphorylation (OXPHOS) pathway, has emerged as another novel target system in drug discovery. New classes of antibacterial proteins interfering with elements of the OXPHOS pathway are highly active in combating dormant or latent mycobacterial infections, showing potential for reducing the period for TB chemotherapy. In 2018, using cryo electron microscopy, Rao’s team determined the high-resolution structure of a mycobacterial respiratory supercomplex, which is the target of a candidate drug in Phase II clinical trial.
The team is also looking at pathogens of other infectious diseases, including avian flu, SARS, hand, foot, and mouth disease, and herpesvirus. “We are keen to uncover the mechanisms underlying these pathogens,” said Rao. “We hope our basic research will lead to new drug discoveries for infectious diseases.”
Cancer Immunotherapy
The ability of the immune system to detect and remove abnormal cells makes immunotherapy an obvious cancer treatment, using approaches including the use of antibodies, vaccines and T-cell infusions.
An immunologist of great experience, Cao Xuetao, president of Nankai University, is a pioneer in cancer immunotherapy and translational research in China. Under Cao’s leadership, The College of Life Science’s Laboratory of Immunity, Inflammation and Cancer is making significant contributions to our understanding of innate signalling in immunity and inflammation, the identification of regulatory immune cell subsets, and the translational research of cancer immunotherapy.
Cancer immunotherapies that inhibit immune checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have been used to successfully treat advanced melanoma, non-small-cell lung cancer, renal cell carcinoma, head and neck squamous carcinoma, Hodgkin’s lymphoma, and bladder cancer.
“A hallmark of cancer immunotherapy is the longevity of patient responses,” says lab member, Xu Xiaoqing. “Memory T-cell formation in patients, the identification of more immune checkpoints, and the development of new strategies in CAR (chimeric antigen receptor) T-cell therapy and cancer vaccine will help significantly improve the effectiveness of cancer immunotherapies.”
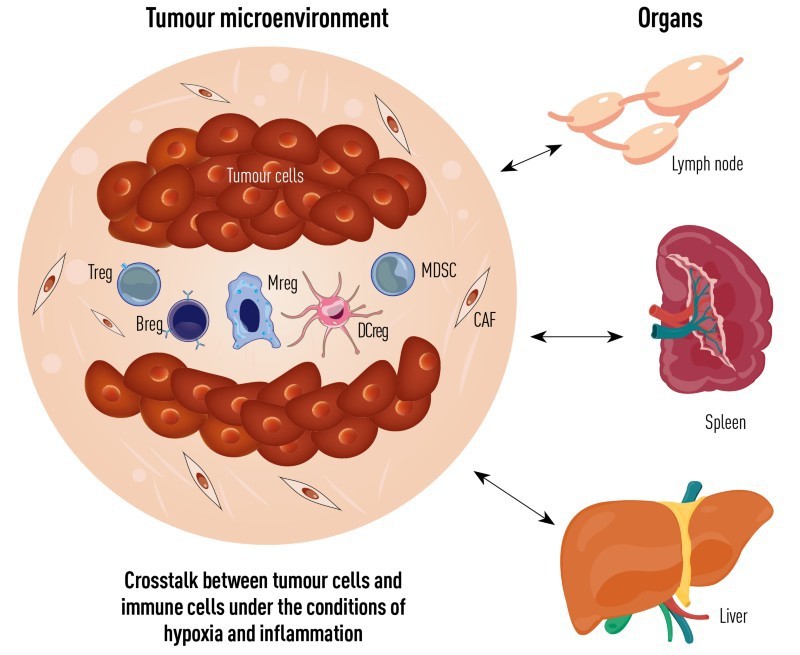
The lab plans to clarify the molecular mechanisms of innate immunity, inflammation, and the mechanisms behind loss of tumour immunity. “We will focus on the process of inflammation resolution, and the crosstalk between cells in the tumour microenvironment and the distinct pre-metastatic organs, particularly, crosstalk between cancer cells and immune cells,” says Cao. “We try to identify the new epigenetic modifiers and metabolic enzymes in inflammation and cancer, aiming to develop new approaches to cancer immunotherapy.”
The team is also looking closely at the innate immune system, the body’s first line of defence against infection. They found that a phosphorylation-mediated IFN-γR2 membrane translocation by E-selectin signalling pathway is required to activate an innate response in macrophages against intracellular bacterial infection. “This indicates that the formation of functional cytokine receptors on cell membranes is an important new layer in innate activation and cytokine signalling,” says Xu.
Cardiovascular tissue regeneration
Nankai researchers are using emerging tissue engineering and nanomedical technologies to design and engineer biomaterials to combat cardiovascular disease, China’s leading cause of death.
These materials need to be biocompatible and bioactive, says Kong Deling, a professor from the Ministry of Education Key Laboratory of Bioactive Materials at Nankai. His team is developing small-diameter artificial vascular grafts. Featuring a novel bi-layered vessel structure, their grafts promote integration, which significantly lowers the risk of blood clots.
Their improvement lies in directing the infiltration of native cells, while modulating macrophages to generate anti-inflammatory M2 phenotypes, an important cell type that predominantly orchestrates tissue regeneration. This optimization leads to rapid endothelialisation, a pivotal mechanism for new blood vessel generation.
Kong’s team has also shown that putting bioactive factors in an elastic material, called polymer poly (l-lactide-co-ε-caprolactone), accelerates several key activities of vascular regeneration. These include endothelialisation (the formation of the tissue lining the inner walls of blood vessels), and the mobilization of native vascular progenitor cells, as well as inducing progenitors to become functional endothelial cells or vascular muscle cells.
In addition, the team has made hydrogels with signalling molecules and short protein peptides that precisely modulate the tissue microenvironment to achieve physiological balance.
Team member, Zhao Qiang, a Nankai professor, has proposed a ‘bump-and-hole’ model that makes it possible to control the catalyzation of nitric oxide prodrugs — drugs that become pharmacologically active after they are metabolized — to release nitric oxide, a blood vessel widening compound, at a specific site.
This technique will contribute to endothelialisation and reduce cardiovascular stress, alleviating conditions such as blood clots and intimal hyperplasia. Animal studies have shown the technique’s efficacy in ischemia and kidney injury, paving the way for future clinical translation.
Another Nankai professor, Yang Zhimou, has developed a new tripeptide regulator, called Nap-FFG, that can selectively self-assemble into hydrogel on to the surface of platelets to block blood aggregation. Along with his colleague at Nankai, Ding Dan, Yang has developed self-assembling, fluorescent peptides that can specifically attach to gram-positive bacteria and efficiently identify bacterial infection. These fluorescent nanoprobes can be used to study disease pathology, for example, by tracking immune cells during the course of inflammation. These engineered materials offer new possibilities for translational medicine.
Cell biology for non-communicable diseases
Breakthroughs in mitochondrial, telomere, and stem cell biology by Nankai’s research teams are improving knowledge on diseases associated with ageing.
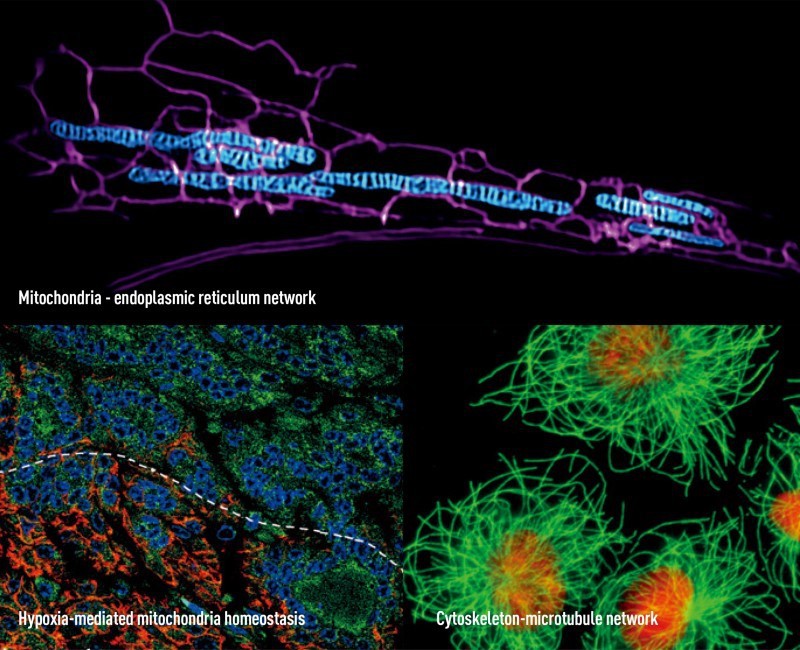
A team led by Chen Quan, the college dean, aims to elucidate how mitochondria influence cell viability and death. Mitochondria typically provide cells with enough energy to function properly, and their disruption may accelerate ageing and lead to diseases. In particular, Chen’s team focuses on the molecular mechanisms of mitochondrial responses to stress. The team is best known for its discovery of FUNDC1, a novel mitophagy receptor responsible for regulating the removal of unwanted mitochondria, uncovering a previously unknown mechanism for mitochondrial quality control. Their screening for small molecules targeting the mitochondrial dynamic contributes to drug development for ageing-related diseases.
Working with Zhu Yushan, Chen has also uncovered a new molecular pathway for autophagy, the ability of cells to break down in a controlled manner. This pathway, known as the ULK1-SRC-mATG9 axis, was found to regulate the initiation of autophagy flux, the abnormality of which has been found in tumours. These breakthroughs outline the fundamental principles of mitochondria and autophagy in tissue homeostasis and tumour progression.
Working on telomeres, DNA structures found at the ends of chromosomes, Liu Lin’s team has determined their role in oocytes and how they regulate embryonic development, cell reprogramming, and pluripotency — the ability of a cell to produce all cell types of the body.
In stem cells, it was previously believed that Erk, a cell signalling pathway, was not central to pluripotency. But, findings by Chen Lingyi’s team have demonstrated it plays an indispensable role and is associated with telomere homeostasis. Furthermore, the team has identified non-genetic modifications of heterochromatin, a packed form of DNA, in ovarian ageing, which is important for developing new strategies for ovarian treatment, preserving fertility, and restoring functionality.
The college also encourages scientists to work on cell dysfunction in diseases. Examples include exploration of cellular and molecular changes in healthy and abnormal hearts, and studies on cell cytoskeletons to further understand why diseased cells often have an abnormal shape. The latter, a work by Zhou Jun’s team, has led to identification of novel microtubule-binding proteins and their roles in cancer progression and virus-host cell interactions.
These explorations by Nankai life scientists have significantly improved our understanding of cell biology and human diseases, as shown by their publications in high-profile journals such as Nature, Nature Cell Biology, and Nature Biotechnology.