New Progress Made by Nankai Research Team in the Structure Analysis of Anti-tuberculosis Drug Targets

Tuberculosis caused by Mycobacterium Tuberculosis is an ancient chronic infectious disease, which humans have been fighting against for quite a long time. The Global Tuberculosis Report 2020 issued by WHO estimates that the number of people latently infected with tuberculosis in the world is close to 2 billion. In 2019, there were 9.96 million new tuberculosis patients worldwide and 1.21 million deaths. Among the newly diagnosed tuberculosis patients worldwide in 2019, about 3.3% of new patients and 18% of retreated patients were resistant to rifampicin. Globally, it is estimated that the number of patients with rifampicin-resistant tuberculosis is about 465,000, of which multi-drug-resistant tuberculosis accounts for about 78%. Drug-resistant tuberculosis has posed a major threat to global public health. Therefore, the discovery of anti-tuberculosis drug targets and the development of new drugs are imminent.
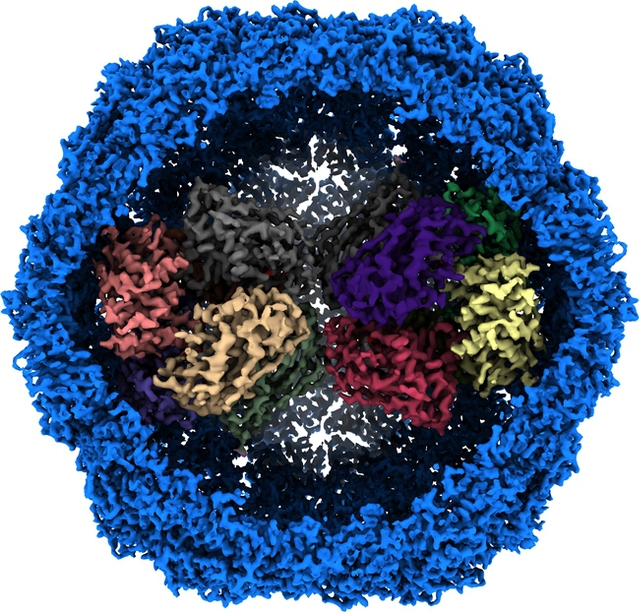
Mycobacterial protein nanocompartment loaded with Dy-P protein
On April 15, Associate Professor Gong Hongri and Academician Rao Zihe from the College of Life Sciences of Nankai University, together with the Shanghai University of Science and Technology and other units, published a paper titled Cryo-EM structure of Mycobacterium smegmatis DyP-loaded encapsulin in the internationally renowned journal Proceedings of the National Academy of Science (PNAS). The research team used single-particle cryo-electron microscopy to successfully decipher the high-resolution three-dimensional structure of a naturally extracted mycobacterial protein nanocompartment loaded with DyP protein for the first time, revealing the molecular mechanism of nanocompartment system to remove hydrogen peroxide and protect cells from oxidative damage. The result provides a structural basis for the development of anti-tuberculosis drugs.

The hydrogen peroxide removing mechanism of DyP-loaded encapsulin
The research team isolated and purified natural nanocompartment protein complexes with a molecular weight of ~2.2 MDa with peroxidase activity from the membrane components of Mycobacterium smegmatis (a model system for M. tuberculosis) at the plateau period, and used single-particle cryo-electron microscopy technology to thoroughly analyze the three-dimensional structure of the nanocompartment loaded with cargo protein for the first time. It is worth mentioning that the respiratory chain super complex III2IV2SOD2 mentioned in the paper published by the research team in Science in 2018 can convert superoxide radicals into H2O2. The spatial distribution and functional synergy between the nanocompartment system and the respiratory chain system suggest that Mycobacterium tuberculosis has stronger antioxidant capacity. In addition, the structural information of the nanocompartment in this study also provides important guidance for the application of protein-based nanoparticles in the fields of drug delivery, nanoreaction chambers, and vaccine development.
Paper URL: https://www.pnas.org/content/118/16/e2025658118
(Reported by Chunhong Lin, translated by Menglin Lu, edited by Daniel Stefan and JianjingYun)









