Partial Clinical Trial Results of Original Anti-glioma Drug of Professor Chen Yue's Team Announced
The original drug candidate ACT001, developed for over 11 years by Professor Chen Yue from the College of Chemistry of Nankai University, is being tested in clinical trials in China, Australia and the United States for malignant glioma, a medical problem worldwide. A number of Chinese and foreign doctors leading the trial have announced the results of the clinical trial of ACT001 at the academic meetings of the American Society of Clinical Oncology (ASCO) and Society of Neuro-Oncology (SNO): the new drug candidate is initially proven to be safe and effective, and one patient with recurrent glioblastoma (GBM) has been treated with monotherapy for over 50 months and has maintained complete remission for over 27 months.
ACT001 is a new structured small molecule compound created by Professor Chen Yue's team. The raw material for its preparation is feverfew chrysanthemum lactone, which was first extracted from the traditional herb feverfew chrysanthemum in western countries. The lactone has a selective killing effect on tumor stem cells, but its content is very low in feverfew. Chen Yue's team has conducted a lot of exploration and finally found that the root bark of the endemic plant Xin Yi (Magnolia delavayi Franch) contains up to 9.6% of feverfew lactone, and the actual extraction rate is stable at 5%~6%. By means of semi-synthesis, the team improved the chemical structure of the lactone and obtained DMAMCL (i.e. ACT001). This small molecule compound can enter the bloodstream by oral and be able to break the blood-brain barrier to act directly on tumor lesions in the brain.
ACT001 has fully independent intellectual property rights and has been granted patents in more than 20 countries. In 2017, it has been certified as an orphan drug by the FDA and EMA in 2017 and conducted clinical trails in Australia, China, and the U.S., of which 6 are phrase 2 or 1b/2a and 2 are phase 1 clinical for pediatric tumors, with indications including glioblastoma, diffuse endogenous pontine glioma, brain metastases, optic neuromyelitis optica and pulmonary fibrosis.
At the ASCO meeting in June, Professor Jason Lickliter from Nucleus Network Hospital in Australia and Professor Wang Ping from Tianjin Medical University Cancer Institute & Hospital presented the clinical trial results of ACT001 for recurrent glioblastoma (GBM) in a poster session.
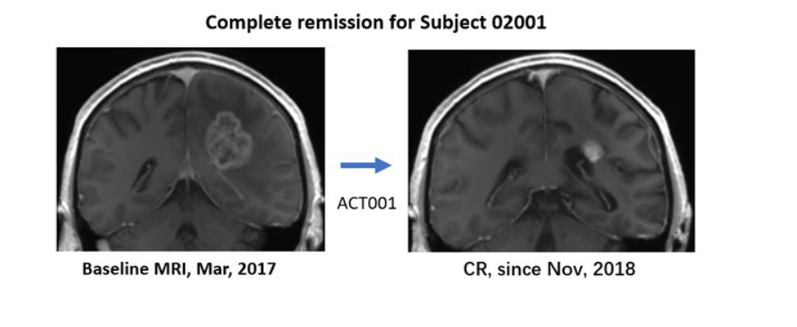
Left: Pre-enrollment MRI image of a patient with recurrent GBM (March 2017) with a nearly 4cm tumor.
Right: MRI image of this patient after ACT001 monotherapy, achieving complete remission since November 2018.
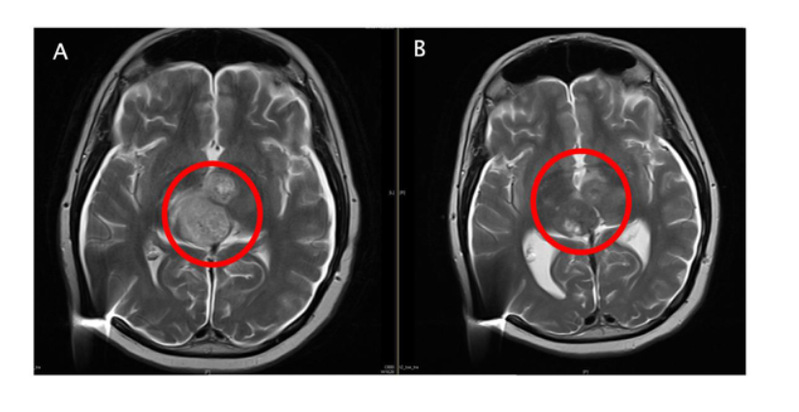
Figure A: Pre-enrollment MRI image with a large number of diffuse lesions shown in the red circle.
Figure B: Post-ACT001 monotherapy image with a significant reduction in tumor cell density.
The potential use of ACT001 is not limited to the treatment of gliomas, Chen Yue said. The team has also found that ACT001 improves the survival rate of animals after radiation and has now applied for a patent for its use in radiotherapy protection. In addition, scientific colleagues are discovering the use of ACT001 in the treatment of various inflammatory diseases, cancer and fibrosis.
It is worth noting that the dosage of clinical trial drug ACT001 has risen sharply, and the demand for feverfew chrysanthemum lactone and Xin Yi (Magnolia delavayi Franch) has also increased substantially. The partner company has set up demonstration bases in several places to promote the development of Xin Yi plantation industry, which supports scientific research and also achieves the good social benefits of helping farmers to increase their income to a certain extent.
Links:
ASCO Conference Poster: https://meetinglibrary.asco.org/record/196587/abstract
ASCO Conference Poster: https://meetinglibrary.asco.org/record/196574/abstract
SNO Conference Report:
https://app.oxfordabstracts.com/events/1809/program-app/submission/233921
Reported by Wu Junhui, edited by Wei Chengjin.









