Nankai Team Achieves Efficient Chemical Synthesis of N-heterocyclic Compounds Using Tense Ring Cross-dimerization and Ring Expansion Reaction
Recently, Zhao Dongbing's team at the State Key Laboratory of Elemento-organic Chemistry of Nankai University has made a breakthrough in the field of synthetic chemistry. The team has proposed a new strategy for the synthesis of N-heterocyclic compounds, which is the first time to achieve an efficient chemical synthesis of several types of N-heterocyclic compounds with important biological activities by using tense ring cross-dimerization and ring expansion reactions. The research results have recently been published in Nature Chemistry under the title of A ring expansion strategy towards diverse azaheterocycles.
Zhao Dongbing introduced their creative conceptual approach to the efficient synthesis of azaheterocycles by cross-dimerization and ring expansion of two small rings. These methods have advantages such as 100% atom economy, the possibility to achieve an efficient synthesis of heterocycles that are difficult to synthesize by conventional methods, and modularity with the easy adjustment of substituent types. However, there are many challenges to the successful implementation of this ring expansion strategy, such as the often highly reactive nature of small ring compounds, which are prone to decomposition or self-polymerization reactions, and the fact that the reaction process involves bond breaking and reorganization, which may lack selectivity, resulting in bonds mismatching and obtaining mixtures, making the reactions impractical.
In this study, we propose for the first time a tense ring cross-dimerization ring expansion strategy to achieve the efficient chemical synthesis of several classes of biologically important diverse azaheterocycles compounds such as acridine, pyridone, and uracil backbones. Zhao Dongbing said.
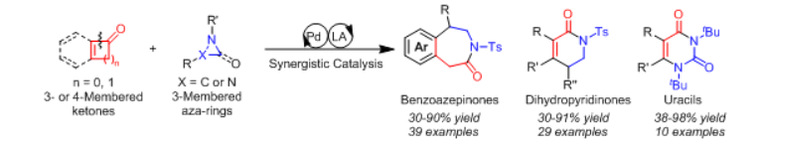
Figure 1: Cross-coupling of three-membered aza rings and three- and four-membered-ring ketones assembling diverse azaheterocycles
Specifically, the researchers cleverly used the concept of palladium/Lewis acid co-catalysis to activate ring ketones (three- and four-membered) with three-membered aza rings separately, and used the addition of nitrogen negative ions to carbonyl groups on the aza-organometallic species formed in the reaction as a linking reaction to structure other metal-catalyzed radical steps to successfully achieve the fusion and ring expansion of two small rings to obtain a variety of acridine, pyridone and uracil backbones (Figure 1). This reaction is flexible, efficient and stereospecific. In the paper, the researchers demonstrated that the use of this new diverse azaheterocycles synthesis strategy can greatly simplify the synthesis of many natural products and important drug molecules by shortening the reaction steps and improving the efficiency of synthesis.
Zhao Dongbing's team has explored the mechanism of the reaction in detail through experiments and DFT theoretical calculations. The reaction is first initiated by the regioselective oxidative addition of the C-C bond to the ring ketone compound by the palladium catalyst, and the resulting ring palladium metal intermediate attacks the nucleophile of the three-membered aza rings in a SN2 manner, and the resulting amine anion then nucleophilically adds to the C=O double bond of the ring ketone compound, and reorganizes the C-C bond and the C-N bond by reductive acylation and reductive elimination processes to obtain the N-heterocyclic compounds, which undergoes a Pd0/II/IV catalytic cycle. Lewis acid can reduce the activation energy required to break the C-C and C-N bonds during the reaction.
Ultimately, the researchers concluded that ring expansion by cross-dimerization of two different tension rings is an important research advancement in synthetic chemistry regarding the synthesis of rings, which is expected to provide new ideas and insights for the synthesis of complex compounds and has the potential to change the inherent logic of inverse synthetic analysis in heterocyclic synthesis. (Contributed by College of Chemistry)
Researcher Zhao Dongbing from the State Key Laboratory of Elemento-organic Chemistry of Nankai University is the corresponding author of the paper; Li Ruirui, a Ph.D. student from Nankai University, is the first author of this paper and has completed the main experimental work; Li Bo, an undergraduate student from Nankai University, has completed the theoretical calculations as the second author.
Information of the paper:
https://www.nature.com/articles/s41557-021-00746-7
Edited by Hao Jingqiu









