Nankai Research Team Makes Progress in the Biochemical Mechanism of Paf1 Complex Promoting Histone H2B Monoubiquitination

The RNA polymerase II-related factor 1 (Paf1) complex is a multisubunit complex that is highly conserved in eukaryotes and consists of Ctr9, Paf1, Leo1, Cdc73 and Rtf1, and the human Paf1 complex also contains an additional subunit, Ski8. The Paf1 complex has been reported to be involved in a variety of cellular life activities and associated with the development of different tumors, and almost all of these processes are associated with post-translational modifications of transcriptionally coupled histones on which the Paf1 complex depends. Among them, histone H2B monoubiquitination is one of the most critical post-translational modifications of transcription-coupled histone. Despite nearly two decades of research, the biochemical mechanism of how the Paf1 complex promotes H2B monoubiquitination is not fully understood.
Recently, Professor Long Jiafu's research team at the College of Life Sciences and the State Key Laboratory of Medicinal Chemical Biology, Nankai University, has made important progress in the study of the biochemical mechanism of Paf1 Complex-induced H2B monoubiquitination, and the related paper titled Biochemical Insights into Paf1 Complex-induced Biochemical Insights into Paf1 Complex-induced Stimulation of Rad6/Bre1-mediated H2B Monoubiquitination was published online in the August 12 issue of PNAS, a journal of the Proceedings of the National Academy of Sciences of the United States of America.
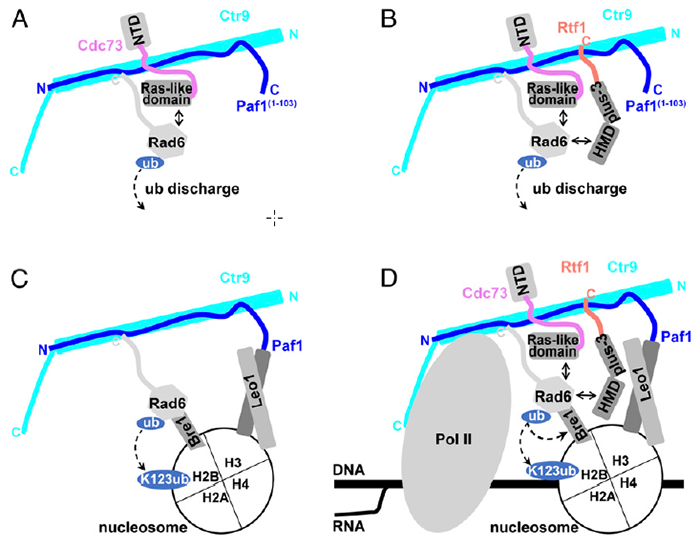
This paper finds that the Paf1 complex enhances the ubiquitin transfer efficiency of Rad6 through multivalent interactions with the ubiquitin-binding enzyme E2 (Rad6) and the substrate nucleosome, thereby promoting H2B monoubiquitination. Biochemically, the Ctr9 subunit, as a backbone protein, directly interacts with the carbon terminal acidic region of Rad6, providing a platform for the Paf1 complex to efficiently and specifically promote H2B monoubiquitination. More importantly, the Ras-like structural domain of the Cdc73 subunit and the HMD structural domain of the Rtf1 subunit enhance the catalytic activity of Rad6 to transfer ubiquitin through direct interaction with Rad6, thereby promoting H2B monoubiquitination. In addition, the Paf1/Leo1 heterodimer specifically recognizes the nitrogen-terminal tail region of the substrate nucleosome histone H3, thereby promoting H2B monoubiquitination. These results reveal the potential biochemical mechanism of how the subunits of the Paf1 complex efficiently synergize to promote H2B monoubiquitination, and lay the foundation for elucidating the complex biological functions of the Paf1 complex.
The team has published papers in Nucleic Acids Research in 2013 and Nature Communications in 2018 to explore the structural basis and functional mechanism of the Paf1/Leo1 subcomplex and Ctr9/Paf1 subcomplex involved in the Paf1 complex assembly, respectively. This achievement is another progress of the team in structural and functional studies of Paf1 complexes.
Link to the paper: https://www.pnas.org/content/118/33/e2025291118









