Nankai Research Team Used Self-Developed Alkenyl-Diamine Rare-Earth Catalytic System to Realize the Silicon-Hydrogenation Reaction of Isomerised Olefins

Rare earth elements
Recently, Cui Chunming's research team of Nankai University realized the hydrosilylation of isomerised olefins (ACS Catal. 2018, 8, 2230) and the double hydrosilylation of isomerised alkynes (Angew. Chem. Int. Ed. 2020, 59, 2365) using an self-developed alkenyl-diamine rare-earth catalytic system. 1,3-enyne contains conjugated olefin and alkyne units, which are easy to prepare, structurally diverse and diverse in reactivities, and are important synthetic building blocks in organic synthesis. However, catalytic in hydrosilylation of 1,3-enyne faces the problem of regioselectivity and stereoselectivity control, and there are three main competing pathways, namely 1,2-, 1,4- and 4,3-addition pathways. However, only a few cases of catalytic 1,4- hydrosilylation reactions of 1,3-enyne have been reported with a very limited range of substrates.
Based on the previous work on rare-earth-catalyzed hydrosilylation, this team has achieved the first highly efficient and selective 1,4-hydrosilylation reaction of branched 1,3-enynes using anionic rare-earth complexes as catalysts to obtain a variety of tetrasubstituted silylallenes under mild reaction conditions. Among them, the radius of rare earth ions and the ate structure play a decisive role in the reaction. In addition, the ligand insertion and σ-bond complex decomposition reaction mechanism of rare earth-catalyzed 1,4- hydrosilylation of 1,3-enyne are proposed by combining deuteration experiments, kinetic experiments and DFT calculations.
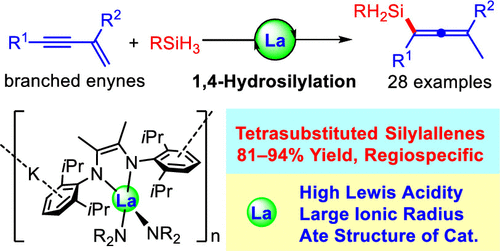
Structure of the rare earth metal catalyst and schematic diagram of the catalytic hydrosilylation reaction
Combining with the team’s previous mechanistic studies, the researchers concluded that the mechanism of the rare earth-catalyzed 1,4- hydrosilylation of 1,3-enyne should be as shown in the figure above. First, the alkenyl-diamine ligand rare-earth diamine potassium salt reacts with silane to produce the anionic rare-earth hydride A. After that, A attacks on the terminal site of the alkynyl double bond to form η3liganded alkynyl-lanthanide intermediate B. Finally, B undergoes σ-bond metathesis with the silane to form silylallenes examples and rare earth hydrides, completing the catalytic cycle.

Theoretical study of the catalytic reaction mechanism: energy diagram of the catalytic mechanism and structures of transition states and intermediates
The researchers concluded that this work has achieved the first selective 1,4-hydrosilylation of alkynes using rare earth metal catalysts, which provides new methods and ideas for the development of new organosilicon reagents and materials.
The research results were published as “Rare-Earth-Catalyzed Selective 1,4-Hydrosilylation of Branched 1,3-Enynes Giving Tetrasubstituted Silylallenes” in the Journal of the American Chemical Society (J. Am. Chem. Soc. 2021, 143, 12913–12918). The corresponding author is Prof. Cui Chunming of Nankai University, with Associate Professor Li Jianfeng as co-corresponding author and Ph.D student Chen Wufeng as first author. The theoretical calculations of this work were assisted by Prof. Xu Xiufang and Jiang Chunhui. The above work was supported by the National Natural Science Foundation of China.









