Nankai Team Revealed that Paraquat Can Spontaneously and Ultrafastly Degrade in Water Microdroplets
Paraquat is a kind of fast sterilant herbicide. From the perspective of poisoning mechanism, it belongs to viologen compounds. Being very stable in nature, viologen is a kind of toxic compounds with the chemical structure of 1,1' – disubstituted 4,4'- bipyridium salt, with a half-life period of 23 weeks in water and of up to 6 years in soil. If released into the environment, it will pose a potential health hazard to surrounding mammals.
Recently, the team of researcher Zhang Xinxing of Nankai University developed a new method for fast, cheap and convenient degradation of viologen compounds in the natural environment. It was found that viologen compounds can spontaneously degrade in micro-droplets by atomizing the aqueous solution of viologen compounds into micron-sized water droplets and using in situ mass spectrometry detection method. The result provides a new thought for the environmental pollution control of toxic and harmful substances. The related paper was published in J. Am. Chem. Soc. Gong Chu, a doctoral candidate of Nankai University, is the first author of the paper, and Nankai University is the only corresponding unit of the paper.
Zhang Xinxing's team found that in the microdroplet reaction system, it only takes tens of microseconds to realize the ultrafast kinetics of spontaneous degradation for viologens. For the spontaneous degradation phenomenon, the relatively unstable viologen radical cation is emerged through the divalent viologen compounds reduced by the electrons spontaneously generated on the surface of microdroplets. Based on this, further decomposition is followed through Beta elimination and Hoffmann elimination reactions. Mass spectrometry provides strong support for the free radicals and intermediates involved in the above reaction mechanism (Figure 1). The capture of free electrons by viologens offers a strong evidence for the spontaneous existence of free electrons and hydroxyl radicals on the surface of microdroplets, which is the most cutting-edge controversial field in the world.
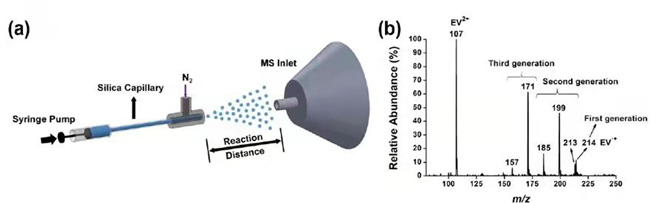
Figure 1. a) Schematic diagram of microdroplet atomising installation; b) Diagram of mass spectrometric analysis for degradation products of ethyl paraquat
The research has been supported by the National Natural Science Foundation of China and the Science Fund for Distinguished Young Scholars of Tianjin. Click for details of the paper: https://pubs.acs.org/doi/abs/10.1021/jacs.1c12028
(Edited and translated by Nankai News Team)









