Nankai Team: Organoboron "Knocks on the door" of Pyridine Drug Synthesis
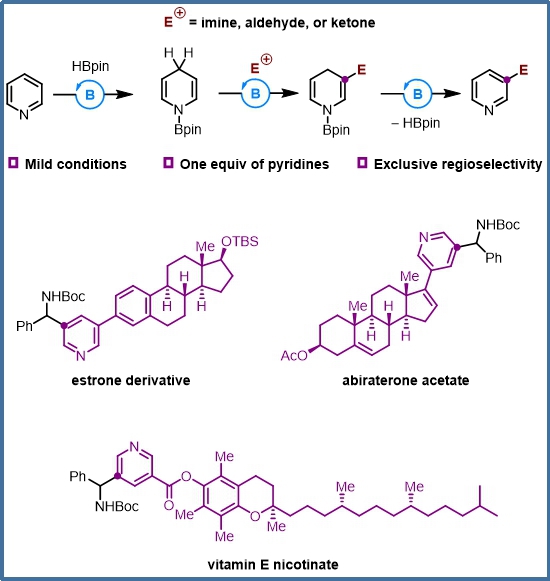
Diagram of the new method of Pyridine C3 modification and its application in drug molecular modification
Pyridine compounds are one of the basic raw materials for pesticide, medicine, daily chemicals and other industries. Recently, Wang Xiaochen’s research group of the College of Chemistry, Nankai University, used organoboron as a catalyst to activate the reaction activity of pyridine C3 position, successfully "Knocking on the door" of the efficient synthesis of pyridine compounds.
Wang Xiaochen's research group has been devoted to the research of organoboron catalysis for a long time, developed two kinds of chiral organoboron catalysts with five-membered-fused ring and five-membered spiro ring as the framework, and completed the tandem reactions and asymmetric tandem reactions of pyridine, etc. Based on the above work, this research group has achieved pyridine C3 alkylation reaction by using the 1, 4-dihydropyridine intermediate generated in the pyridine borohydride reaction catalyzed by organoboron and introducing electrophilic reagent. The strong nucleophilicity of β position of 1, 4-dihydropyridine ensures high activity and specific regioselectivity of the substitution reaction.
"In simple terms, we have realized the difficult pyridine dearomatization by utilizing the high activity of organoboron catalysis hydroboration, making its electronic structure changed. It’s equivalent to the injection of electrons, which significantly enhances the electron cloud density of C3 and activates the reactivity of C3", Wang Xiaochen said.
Experimental results show that the new strategy of pyridine meta functionalization established by Wang Xiaochen's research group is applicable for all kinds of substituted pyridines and imines, and can be directly applied to the modification of multiple drug molecules, as well as various electrophiles such as aldehydes, ketones and eschenmoser salts, etc.
"What’s more noticeable is that the catalytic reaction under the new strategy requires a small amount of pyridine, only for one equivalent, with specific regioselectivity and mild reaction conditions, no more than 80°C. It can be said that the new method provides a convenient, efficient, accurate and universal new way for the post-modification of pyridine-contained drug molecules", Wang Xiaochen said.
A few days ago, the paper describing this work was published online in the international academic journey—Journal of the American Chemical Society(JACS). This work has been supported by such projects and units as the Key R&D Program of the Ministry of Science and Technology of the People’s Republic of China, the National Natural Science Foundation of China, Tianjin Municipal Science and Technology Bureau, and Haihe Laboratory of Sustainable Chemical Transformations, etc.
Paper link: https://pubs.acs.org/doi/abs/10.1021/jacs.2c00962
(Edited and translated by Nankai News Team)









