NKU Team Makes Key Progress in the Field of Nitric Oxide-regulated Regeneration of Artificial Vascular Tissues
Recently, the research group headed by Prof. Zhao Qiang from the State Key Laboratory of Medicinal Chemical Biology of Nankai University made new progress in the preparation of new bio-hybrid artificial blood vessels and regulation of their tissue regeneration. The team put forward a new strategy for artificial blood vessels to improve tissue regeneration and restructuring through local release of nitric oxide (NO), and designed and prepared bio-hybrid artificial blood vessels with NO slow-release function. This vessel can generate NO in the in vivo environment, effectively regulate endogenous vascular stem/progenitor cells, promote vascular tissue regeneration and inhibit angiosteosis. The research findings were published on Cell Reports under the title "Nitric oxide impQroves regeneration and prevents calcification in bio-hybrid vascular grafts via regulation of vascular stem/progenitor cells".
Led by Prof. Zhao Qiang, a team of researchers designed a bio-hybrid artificial blood vessel with the function of nitric oxide slow-release by combining the natural extracellular matrix with electrospinning-based artificial blood vessels. This vessel has a bilayer structure: the inner layer is a great saphenous vein of pigs treated with decellularization, which provides good biocompatibility and regenerative activity. The outer layer uses the functional material of nitrate developed by the research team, which provides mechanical support. The mechanical strength and the artery burst pressure of composite blood vessels can reach or approach that of natural arteries. Furthermore, nitrate materials can be transformed into NO through multiple reactions in the in vivo environment. Under the mouse and rabbit models, NO locally released by composite artificial blood vessels can effectively improve regeneration of vascular tissues, promote endothelial formation, and inhibit pathological vascular remodelings such as intimal hyperplasia and vascular calcification, thereby significantly improving long-term vascular patency.
The research team used lineage tracing technology as well as in vitro cell experiments to investigate the key role and regulatory mechanism of endogenous vascular stem/progenitor cells (SPCs) in the regeneration and remodeling of vascular tissues. According to the findings, NO plays a role in facilitating vascular regeneration and inhibiting vascular calcification by regulating SPC differentiation in the direction of vascular endothelial cells and inhibiting osteogenic differentiation. Further studies revealed that NO has a bidirectional regulatory effect on the osteoblast differentiation of SPC, and low0concentration (nmol-μmol) NO can inhibit the osteoblast differentiation of SPC by regulating two-thirds of signaling pathway of TGF-pSMAD, thereby reducing vascular calcification. High-concentration NO (mmol) plays an opposite role. These findings abundantly demonstrate that NO plays a key role in regulating vascular stem/progenitor cells and facilitating vascular tissue regeneration. Bio-hybrid artificial vessels with NO slow-release function have good prospects of clinical use.
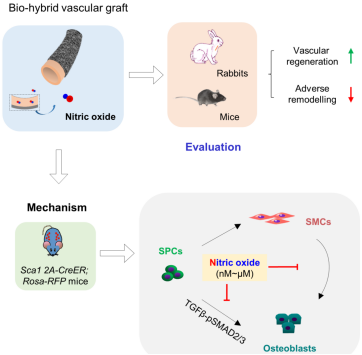
Preparation and evaluation of bio-hybrid artificial vessels with slow-release nitric oxide as well as in vivo regeneration mechanism
Prof. Zhao Qiang, from the School of Life Sciences, Nankai University and the State Key Laboratory of Medicinal Chemical Biology, and Prof. Xu Qingbo, from the School of Medicine, First Affiliated Hospital of Zhejiang University, are the joint corresponding authors of this paper. Wang Fei, Qin Kang and Wang He, doctoral students from the School of Life Sciences, Nankai University, and Associate Professor Wang Kai are the co-first authors of this paper. The project was funded by the National Natural Science Foundation of China (81925021, 81921004, 81871500) and the Tianjin Natural Science Foundation (18JCJQJC46900). (Courtesy of the School of Life Sciences).
Link to the paper: https://www.cell.com/cell-reports/fulltext/S2211-1247(22)00767-7
(Edited and translated by Nankai News Team)









