NKU Scholars Proposed the Method of Electrochemical Transformation of Limestone into Calcium Hydroxide and Valuable Carbonaceous Products for Decarbonizing Cement Production
Recently, the research team headed by Professor Jingshan Luo of the Institute of Photoelectronic Thin Film Devices and Technology, College of Electronic Information and Optical Engineering of Nankai University, proposed a proof of concept for electrochemical transformation of limestone (CaCO3) into calcium hydroxide (Ca(OH)2) and valuable carbonaceous products for decarbonizing cement production based on their research experience in electrochemical water splitting and carbon dioxide (CO2) reduction reaction in view of the problem of large CO2 emissions in cement production. Distinct from the traditional cement production process, in which the high-temperature pyrolysis of limestone releases CO2 while obtaining calcium oxide (CaO), this method does not emit CO2 but converts the carbon element in limestone into valuable carbonaceous products, which can be used as fuel and chemicals. It was expected to be used to decarbonize cement production and help to realise the goals of carbon neutrality and carbon peaking. The research findings were published in the international academic journal iScience under the title “Electrochemical transformation of limestone into calcium hydroxide and valuable carbonaceous products for decarbonizing cement production”.
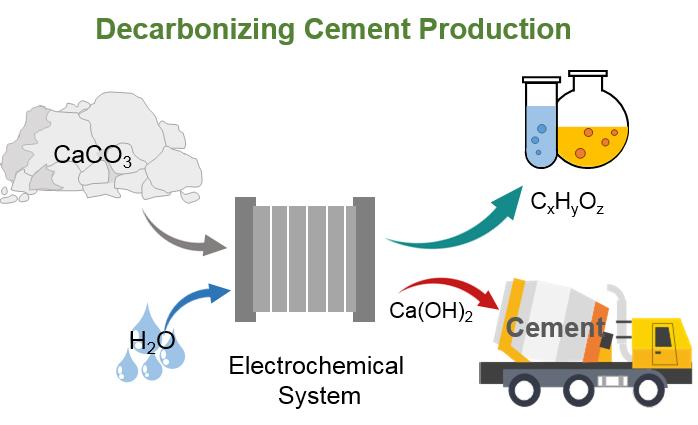
Schematic diagram of electrochemical transformation of limestone (CaCO3) into calcium hydroxide (Ca(OH)2) and valuable carbonaceous products.
In 2021, Nankai University forged strategic cooperation with the Wuhu Municipal People’s Government of Anhui Province and Conch Group. Professor Jingshan Luo, as the project leader, and Santan (Anhui) Science and Technology Institute Co., Ltd., a wholly-owned subsidiary of Conch Group, jointly established Nankai University-Conch Group Joint Laboratory for “Comprehensive Utilization of Carbon Dioxide Resources”, which focuses on exploring the ways of using CO2 as a resource and reducing CO2 emissions in the cement industry.
Based on the previous research on the electrochemical transformation of CaCO3 into Ca(OH)2, and the research experience in electrochemical water splitting and CO2 reduction reaction, Luo’s group proposed a proof of concept for electrochemical transformation of limestone into Ca(OH)2 and valuable carbonaceous products. Distinct from the high-temperature pyrolysis of limestone, this method does not emit CO2 but converts the carbon element in limestone into valuable carbonaceous products, which can be used as fuel and chemicals, providing a novel idea for CO2 emission reduction in the cement industry.
Firstly, this study converts CaCO3 based on a neutral water splitting system. In this process, hydrogen ions (H+) generated by the oxygen evolution reaction in the neutral water splitting process were used to react with CaCO3 to generate calcium ions (Ca2+) and CO2, and Ca2+ bonds with hydroxide (OH-) generated in the system to result in Ca(OH)2, which can be directly used in cement production. Secondly, the carbon dioxide generated in the system is in-situ converted into valuable carbonaceous products by switching the applied voltage, such as carbon monoxide, methane, olefins, etc. The products of this process can be regulated by switching catalysts.
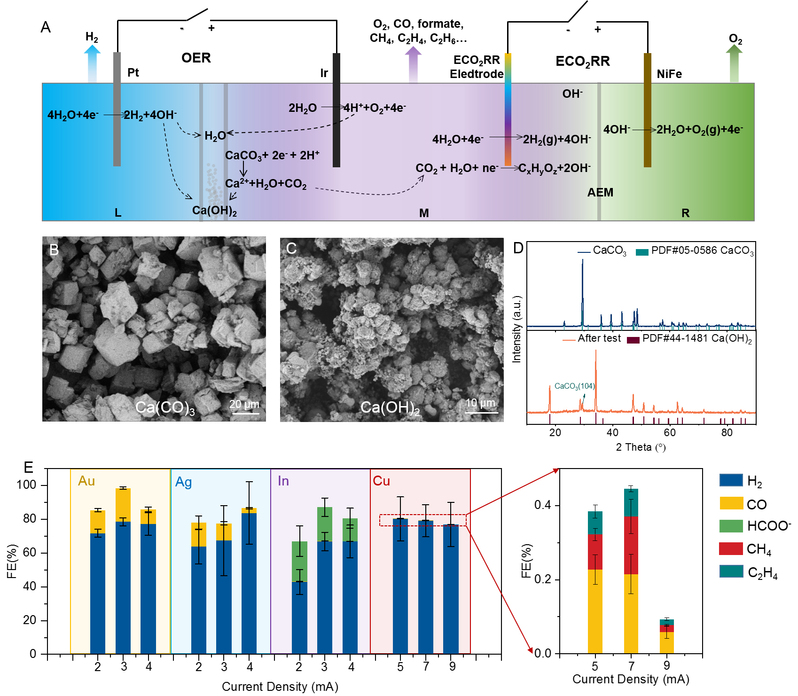
(A) Schematic illustration of the electrochemical transformation of limestone (CaCO3) into calcium hydroxide (Ca(OH)2) and carbonaceous products in an electrolytic cell system; (B) scanning electron microscope images of CaCO3 and (C) Ca(OH)2 ; (D) X-ray diffraction pattern of CaCO3 and Ca(OH)2; (E) The Faradaic efficiency (FE) of valuable carbonaceous products with different metal electrodes.
Nankai University and Conch Group jointly applied for a national invention patent for the technology reported in this paper. The first author of the paper is Dr. Qixian Xie, and the corresponding author is Professor Jingshan Luo.
(Edited and translated by Nankai News Team)









