National Science Review: Zeolite-encaged Mononuclear Copper Centers Catalyze CO2 Selective Hydrogenation to Methanol
Recently, the research findings entitled “Zeolite-encaged mononuclear copper centers catalyze CO2 selective hydrogenation to methanol” by Nankai University was published in National Science Review. Drs. Chai Yuchao and Qin Bin from the College of Chemistry of Nankai University, are co-first authors, and professor Li Landong is the corresponding author. Zeolite-encaged mononuclear copper ions (Cu@FAU; Fig. 1) was designed and constructed, and heterolytic activation of hydrogen molecule and subsequent CO2 were achieved with {Cu-O} Lewis pairs from Cu ions and adjacent framework O atoms. Excellent catalytic activity, high methanol selectivity (89.5%) and good catalytic stability were obtained at the same time at relatively low reaction temperature of ~513 K. The performance of Cu@FAU is at the top level among Cu-based catalysts, showing great potential for industrial application.
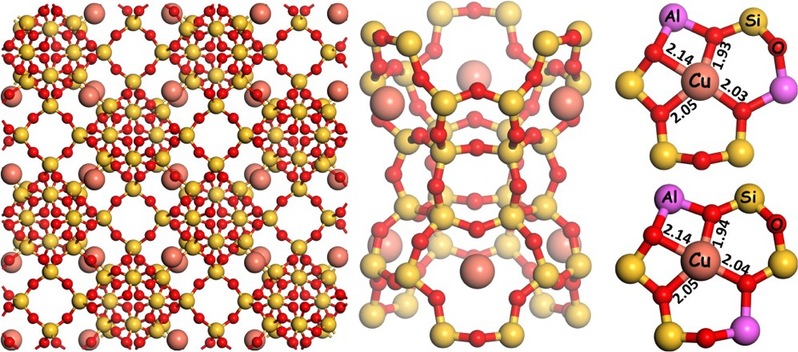
Fig. 1 Structure of Cu@FAU and local coordination environment of isolated Cu ions
The reaction pathway of stepwise CO2 hydrogenation to methanol was interpreted via the combination of in-situ spectroscopy, kinetic analysis and theoretical simulations. The intermediate species of -HCOO, -HCOOH, -CH2O, and -CH3O (Fig. 2) were involved in the process. It is revealed for the first time that zeolite-encaged mononuclear copper centers can catalyze the complex CO2 selective hydrogenation to methanol, involving the activation of three dihydrogen molecules, namely CO2 adsorption-assisted dihydrogen heterolytic activation, HCOOH adsorption-assisted dihydrogen heterolytic activation and CH2O adsorption-assisted dihydrogen heterolytic activation. The research results of this study can provide some new ideas for the understanding of active center of Cu-based methanol synthesis catalysts and the rational design of new catalysts for CO2 selective hydrogenation.
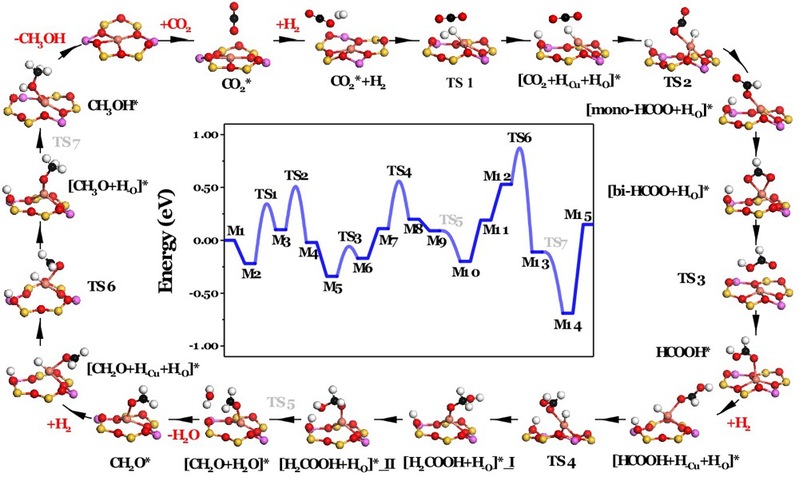
Fig. 2 Reaction pathway of CO2 selective hydrogenation catalyzed by Cu@FAU









