Nankai Team Reveals Macrophages’ Capacity of migration on Non-adherent Substrates in Mesenchymal Mode by Photolithography-based Patterning Manipulation
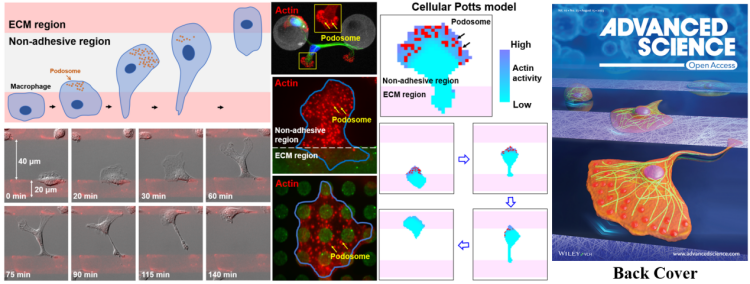
The team led by Professor Pan Leiting and Professor Xu Jingjun of the School of Physics, Nankai University and the TEDA Institute of Applied Physics employed photolithography-based patterning technology to construct an alternate adhesive-nonadhesive substrate consisting of the non-adherent regions based on protein-repelling regent (PEG) and the adherent regions based on extracellular matrix protein (Fibronectin, FN). It was found that primary mouse macrophages can migrate on this alternate adhesive-nonadhesive substrate in the unexpected mesenchymal mode. More than 20 other types of cells were tested, including adherent cells such as MDCK cells and NIH 3T3 cells, cancer cells such as MDA-MB-231 cells and Hela cells, and primary cells such as neutrophils, astrocytes, and microglia, but no such capability was found. Macrophages, as an important innate immune cell in the body, are primarily responsible for pathogen phagocytosis, antigen presentation, etc. Macrophages’ strong migration ability in a non-uniform substrate may be of great significance for their chemotaxis migration in the complex body environment (such as non-adherent areas caused by burns in the body) and play the role of immune functions. The findings were published as a Back Cover paper entitled “Mesenchymal migration on adhesive-non-adhesive alternate surfaces in macrophages” in Advanced Science, a well-known comprehensive journal of Wiley Publishing Group.
Using the alternate adhesive (FN) - nonadhesive (PEG) substrate constructed by photolithography, the team observed that macrophages crossed non-adhesive PEG areas up to 80 μm wide (much larger than cell size) in a mesenchymal mode (outspread leading edge and elongated tail) at a rate of ~1 μm/s in random directions. Interestingly, macrophages need to adhere to the FN area before they can migrate to the PEG regions. Cells on the PEG region are spherical without the ability to adhere or migrate. Experiments such as scanning electron microscopy and local mechanical stimulation further confirmed that macrophages adhere tightly to PEG regions that other cells cannot adhere to. In order to find out the internal mechanism of this powerful migration capacity, the team employed immunofluorescence and STORM single-molecule localization super-resolution imaging techniques and found that podosome, an actin-rich adhesive structure, is specifically enriched in the PEG regions. Dynamic imaging of living cells reveals that podosomes are rapidly generated when cells spread and migrate to the PEG regions, and disappear when the leading edge touches another FN region. Moreover, the uropod tail detached from the initial FN region. Drus and siRNA intervention can inhibit the generation of podosomes, further demonstrating that macrophages’ robust adhesion and migration capacity relies on podosomes. The density of podosome can be upregulated by Myosin IIA inhibition through Blebbistatin and Y27632, thereby significantly enhancing the migration capacity of macrophages. Conversely, inhibition of podosome generation by upregulating Myosin’s activity through PGE2 and CalyA can reduce macrophage’s migration capacity.
Finally, the team built the Cellular Potts model based on the Ising model. Based on framework of statistical physics, this model breaks down a single cell into a collection of two-dimensional grids, which can show the cell shape characteristics, thereby exploring cell migration characteristics in a bottom-up manner. It employs parameters including basal spatial heterogeneity, cytoskeletal polymerization dynamics and podosome turnover dynamics to construct a positive signal feedback loop model. That is, podosome promotes local activation of cytoskeleton, and podosome can be easily generated near the highly active skeleton, which saves the polarizability of model cells in the PEG region and allows the model cells to overcome the energy obstacle on the PEG region. It successfully reproduces the distinctive migration behavior of macrophages on the alternate adhesive-nonadhesive substrate. It was theoretically predicted that macrophage migration would be enhanced on narrower FN stripes, which is verified by further experiment, indicating the rationality of the modeling. To sum up, this study reveals the powerful migration capacity of macrophages as innate immune cells, and also broadens people’s understanding of cell migration.
The team led by Professor Pan Leiting and Professor Xu Jingjun has been committed to interdisciplinary scientific research in cell imaging and manipulation. In terms of cell manipulation, the team developed and improved the photolithography-based patterning technology derived from the semiconductor industry to realize the micrometer-scale manipulation of cells, and conducted research on new methods of intercellular behavior. For example, the team designed concentric ordered patterns, which visibly, directly and quantitatively confirm the existence of signal relay amplification and transmission mechanism in discrete intercellular calcium wave communication, expanding the scope of signal transmission, and improving signal transmission rate and efficiency (ACS Applied Materials & Interfaces, 2018, 10: 2937); They also designed a dumbbell-like pattern to achieve stable regulation of the length of the narrow intercellular bridge at the end of mitosis, and revealed that there are active and passive transmission mechanisms for intercellular bridge-mediated intercellular calcium wave communication in conjunction with theoretical modeling (Biophysical Journal, 2020, 118: 11196); They used circular patterns to reveal that actin retrograde can induce cells to produce a secondary ring structure (Journal of Innovative Optical Health Sciences, 2023, 16: 2244005), and so on.
The first author of this paper is postdoctor Xing Fulin, and the corresponding author is Professor Pan Leiting. Nankai University is the first institution. The research work was also guided by Chen Liangyi, Professor at College of Future Technology of Peking University, and Zhou Jun, Professor at College of Life Sciences, Nankai University.
Link:https://onlinelibrary.wiley.com/doi/full/10.1002/advs.202301337
(Edited and translated by Nankai News Team.)









