NKU Team Published Major Breakthrough in Inorganic Synthesis and Coordination Chemistry in Science
The latest findings of research by Nankai University were published online in Science, on November 17. The study, entitled “An all-metal fullerene: [K@Au12Sb20]5-”, reported the synthesis and bonding mechanism of all-metal fullerene [K@Au12Sb20]5- (Figures 1-2), demonstrating a new compound synthesis technology and the application of precise regulation of metallic bonds in structural chemistry. It offers a refreshingly novel idea for the creation of new materials. This groundbreaking achievement highlights the important position of Nankai University in the field of synthetic chemistry and coordination chemistry of main group metal elements.
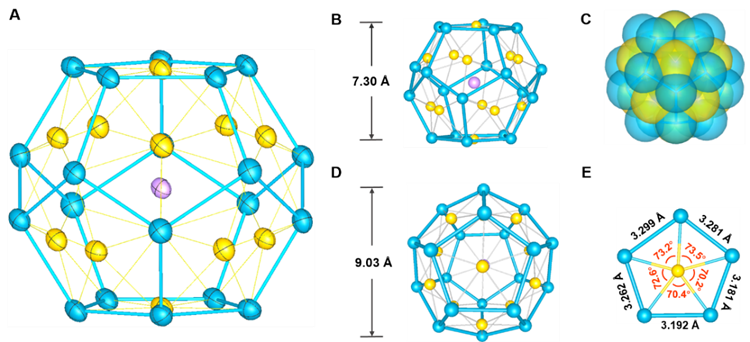
Figure 1: Schematic diagram of cluster structure of all-metal fullerene [K@Au12Sb20]5-, with purple denoting potassium atoms, yellow denoting gold atoms, and blue denoting antimony atoms

A research group led by Dr. Zhong-Ming Sun, a professor at the School of Materials Science and Engineering of Nankai University, successfully prepared all-metal fullerenes [K@Au12Sb20]5- by developing a new synthesis method that combines high-temperature solid-phase synthesis with organometallic chemistry.
The title compound exhibits a structure that approximates to that of an Archimedean dodecahedron, with each side composed of an antimony pentagonal plane that contains one gold atom and an inner diameter of approximately 0.90 nanometers, slightly larger than the diameter of a C60 molecule (0.71 nanometers). Only one potassium cation is embedded in this large cavity of the cluster, which does not need the protection of organic ligands, while its structure can still maintain good chemical stability. This makes it the pure inorganic compound closest to fullerene in the coordination environment to date.

Figure 2: Bonding modes and molecular orbital of [K@Au12Sb20]5- cluster
On the one hand, the stability of this exposed heavy metal globular cluster is achieved as a result of the central potassium ion acting as a template support. On the other hand, the peculiar heterogenous metallic bond between gold and antimony plays a crucial role in maintaining the overall structural integrity (Figure 2).
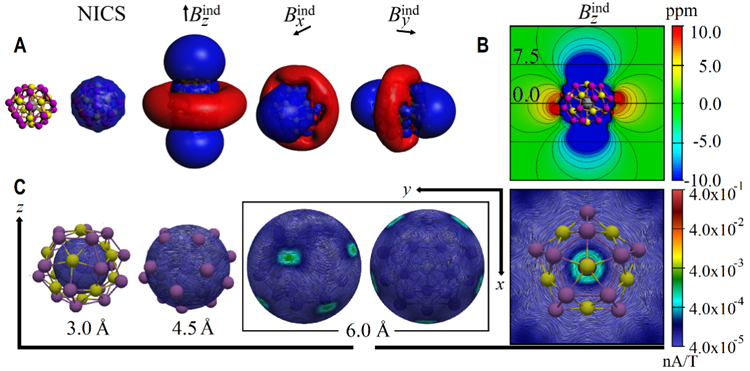
Figure 3: Electronic structure analysis of [K@Au12Sb20]5-
Theoretical calculation reveals that one of the most salient features of the molecule is its 3D spherical aromatic electronic structure, which forms a delocalized π electron cloud on the surface of the cluster. It endows the all-metal fullerene compounds with unique physical characteristics (Figure 3). This discovery is expected to play an important role in optoelectronic materials, room-temperature catalysis and other fields, with a promising application potential.
Chemical & Engineering News reported that the inorganic fullerene might assist chemists in crafting and synthesizing other nanostructures with a precise structure. Professor Andreas Schnepf, a chemist at the University of Tübingen in Germany, said that such molecule has eye-catching bonding properties, adding that these clusters may exhibit interesting reactivity and application potential in solution.


The Zhong-Ming Sun group has been committed to studying synthetic chemistry and coordination chemistry of main-group metal elements. The publication of research results on the synthesis and bonding mechanism of all-metal fullerene [K@Au12Sb20]5- symbolizes the important progress and breakthrough made by Nankai University in this research field.



Link:
https://www.science.org/doi/full/10.1126/science.adj6491
(Edited and translated by Nankai News Team.)









