Innovative Drug developed by NKU Team Approved for Clinical Trials
On March 25th, CP0119 tablets, a class-1 new chemical drug independently developed by a team from the College of Pharmacy of Nankai University, received the Notice of Approval for Drug Clinical Trials issued by the National Medical Products Administration, which granted approval for clinical trials to treat slow transit constipation. This project marks the second new drug project approved for clinical trials by Nankai University as the applicant, following the acquisition of the first clinical trial approval received in 2017.
Epidemiological surveys show that the incidence rate of slow transit constipation in China ranges from 7.3% to 20.39%, and has been showing an upward trend in recent years. However, the existing treatment options and their effects are limited, and the available therapeutic methods often fail to meet expectations. The treatment of slow transit constipation remains a significant challenge. Therefore, it is of great significance to develop a highly effective and low-toxicity therapeutic drug.
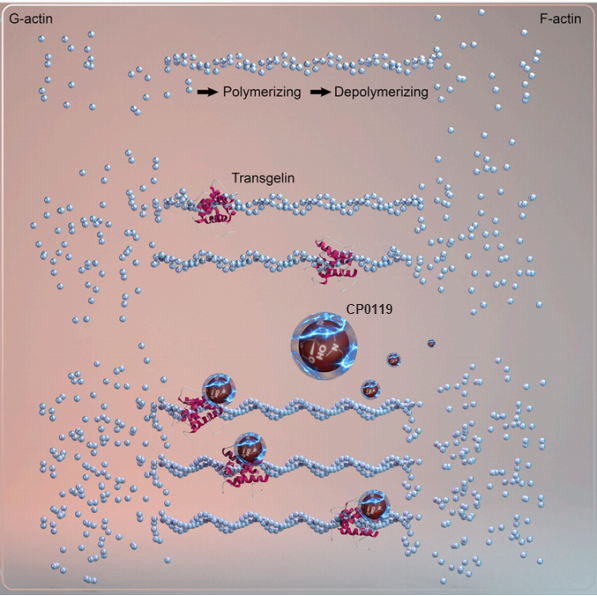
The mechanism of action of CP0119 targeting Transgelin in the treatment of slow transit constipation
Based on this, the Dean of the College of Pharmacy of Nankai University has spearhead the establishment of the CP0119 innovative drug R&D team, with professors Zhou Honggang, Sun Tao and Yang Guang as project leaders. According to the project leads, CP0119 is a novel small-molecule agonist the smooth muscle protein Transgelin, which is an actin-binding protein in the calmodulin family, with a relative molecular weight of 22 kDa. It primarily exists in smooth muscle cells. Concurrently, CP0119 can active Transgelin on intestinal smooth muscle cells, promote the polymerization of G-actin into F-actin, enhancing the formation of stress fiber bundles in intestinal smooth muscle cells, this, in turn augments the cells’ contractile capabilities, stimulates bowel movement, and ultimately achieve the effect of improving slow transit constipation. There is currently no similar drug targeting Transgelin for the treatment of constipation on the market, making the successful development of CP0119 a groundbreaking class-1 innovative chemical drug with a novel target.
Associate Professor Xiaoyu Ai and teacher Xiaoting Gu, key members of the project team, are primarily responsible for promoting the preclinical research of the project. It took four years for the project to complete the pilot scale-up of active pharmaceutical ingredients, formulation development, systematic pharmacological and pharmacokinetic research, as well as standardized preclinical GLP safety evaluation. This comprehensive process has amply proved that CP0119 is of controllable quality, safe and effective. Therefore, CP0119 is expected to become a new therapeutic option for patients suffering from slow transit constipation, offering high economic and social benefits. The results of the project have also been highly praised by clinical experts ,including Bangmao Wang, director of the Department of Gastroenterology, Tianjin Medical University General Hospital.
The development of the CP0119 project is undertaken by the innovative drug R&D team of the College of Pharmacy of Nankai University and the State Key Laboratory of Medicinal Chemical Biology. It is also a significant collaborative achievement with National Institute of Pharmaceutical R&D Center of CR Pharma and Tianjin International Joint Academy of Biomedicine.
(Edited and translated by Nankai News Team.)









