NKU Team’s Photolithographic Micropatterning Manipulation Reveals Collective Cell Radial Ordered Migration
Collective cells are an important active matter system, and their ordered migration plays a key role in life processes such as embryonic development, tissue morphogenesis, and cancer invasion. Under normal physiological conditions, collective cells are often in a confined spatial environment, which regulate the dynamic spatiotemporal evolution of collective cell behavior from the top down, thus affecting the differentiation and fate of cell tissues. Nowadays, emerging technologies represented by photolithographic micropatterning are being applied to precise spatial manipulation of cells in vitro. Under this top-down regulation strategy, a variety of collective cell migration behaviors emerge at the supracellular scale.
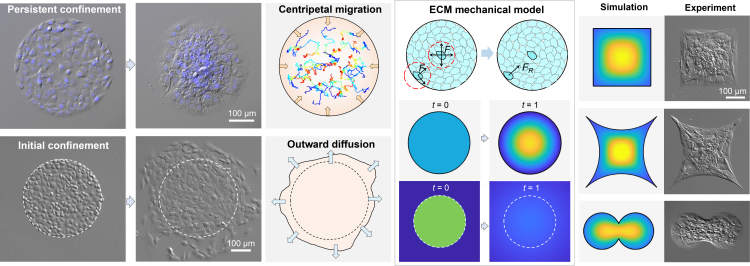
Recently, a team led by Pan Leiting and Xu Jingjun, professors at School of Physics and TEDA Institute of Applied Physics of Nankai University, employed photolithographic micropatterning technology to confine NIH3T3 fibroblasts in a circular substrate, and found that collective cells showed radial ordered centripetal migration, which was markedly different from the widely reported tangential ordered rotational motion of MDCK epithelial cells. It was also pointed out that the radial ordered migration was triggered by the combination of top-down spatial constraint regulation and bottom-up cell-extracellular matrix (ECM) interaction. It offers a new research perspective for exploring the ordered behavior of the active matter system under spatial constraints. The findings were published under the title of “Collective cell radial ordered migration in spatial confinement” in Advanced Science, a comprehensive journal by Wiley Publishing Group.
Recently, a team led by Pan Leiting and Xu Jingjun, professors at School of Physics and TEDA Institute of Applied Physics of Nankai University, employed photolithographic micropatterning technology to confine NIH3T3 fibroblasts in a circular substrate, and found that collective cells showed radial ordered centripetal migration, which was markedly different from the widely reported tangential ordered rotational motion of MDCK epithelial cells. It was also pointed out that the radial ordered migration was triggered by the combination of top-down spatial constraint regulation and bottom-up cell-extracellular matrix (ECM) interaction. It offers a new research perspective for exploring the ordered behavior of the active matter system under spatial constraints. The findings were published under the title of “Collective cell radial ordered migration in spatial confinement” in Advanced Science, a comprehensive journal by Wiley Publishing Group.
The radial ordered migration behavior of the collective NIH3T3 fibroblasts depends on persistent spatial confinement. If spatial confinement disappears, the cells would exhibit an outward diffusion movement instead of centripetal migration. The team developed several data analysis methods to track the movement trajectory of individual cells, and analyzed the movement characteristics of individual cells based on the mean square displacement and directional index of cell movement. It was found that the cells gathered to the center of the pattern in a weak-oriented, diffusive-like manner. Furthermore, analytical tools such as the average radial velocity heat map and the spatiotemporal kymograph of radial order parameters were developed, and it was revealed that the migration dynamics of collective NIH3T3 cells presented radial orderliness and spatiotemporal heterogeneity at the supracellular scale. Specifically, the trend of centripetal migration first appeared at the constraint boundary, and then propagated to the center of the pattern, exhibiting a radial ordered wavefront. The wavefront velocity is significantly higher than the radial velocity of the cell, indicating that the wavefront serves as a long-range signal transmission mechanism at the supracellular level, guiding the way of cell locomotion towards the center, eventually forming global cell centripetal migration. Subsequently, the team employed immunofluorescent staining technology and changed culture conditions to explore the bottom-up cellular biochemical mechanism of centripetal migration of NIH3T3 cells, and discovered that cell-ECM interaction is a key intrinsic factor in this process. The team developed a continuum model based on ECM force, which reproduces the results such as ordered centripetal migration and radial ordered wavefront observed experimentally. It was further pointed out that the formation of the centripetal velocity field depends only on the confinement itself, and is irrelevant to the geometric characteristics of the pattern. Experiments of other patterns also confirmed this theoretical prediction. In conclusion, this study expands understanding of the behavior pattern of ordered migration of collective cells in a confined environment, and also inspires design ideas for in vitro fabrication of artificial tissues and organoids.
The first author of this paper is Dong Hao, a doctoral student, the corresponding author is Professor Pan Leiting, and Nankai University is the first author affiliation.
URL:
https://onlinelibrary.wiley.com/doi/10.1002/advs.202307487
(Edited and translated by Nankai News Team.)









