NKU Team Develops Efficient Electrocatalyst for Hydrogen Production via Water Electrolysis
A significant milestone in the research of water electrolysis for hydrogen production has been made by Professor Jingshan Luo’s team from Nankai University’s College of Electronic Information and Optical Engineering, in partnership with Professor Federico Calle-Vallejo’s team from the University of the Basque Country, Spain. The collaborative team utilized metal-support interactions to develop a high-activity hydrogen evolution catalyst in an alkaline environment. This catalyst can operate stably at 5 A cm-2 for over 1,000 hours. This accomplishment addresses the commercial application for hydrogen production via anion exchange membrane (AEM) water electrolysis. The research outcomes have been published in the prestigious journal Nature Communications.
Currently, two main technologies dominate the field of water electrolysis for hydrogen production: alkaline water electrolysis (ALK-WE) and proton exchange membrane water electrolysis (PEM-WE). ALK-WE is known for its low production cost and mature industrialization but is limited by low hydrogen purity and energy efficiency. In contrast, PEM-WE offers higher energy efficiency and produces hydrogen with greater purity, but its reliance on precious metal catalysts for operation in acidic conditions leads to higher production costs. Anion exchange membrane water electrolysis (AEM-WE) is regarded as a third-generation solution that merges the strengths of both ALK-WE and PEM-WE. It offers advantages such as high efficiency, low cost, and fast start-up and shut-down. However, its commercial application is hindered by the limited stability of electrolyzer systems under high current densities. As a result, one of the key challenges for AEM-WE is developing alkaline hydrogen evolution catalysts that maintain long-term, stable performance at high current densities.
Jingshan Luo explains that current platinum-based materials are commonly used as catalysts in hydrogen evolution reactions due to their excellent performance, albeit at a high cost. Ruthenium, a less expensive precious metal with high catalytic activity and strong durability, is emerging as a promising alternative to platinum with significant potential for application.
“Most of the reported ruthenium-based hydrogen evolution catalysts for alkaline conditions have been tested at low current densities. Our team is focusing on the critical challenge of maintaining high performance at high current densities, which is essential for the large-scale commercial application of these electrocatalysts,” said Professor Jingshan Luo.
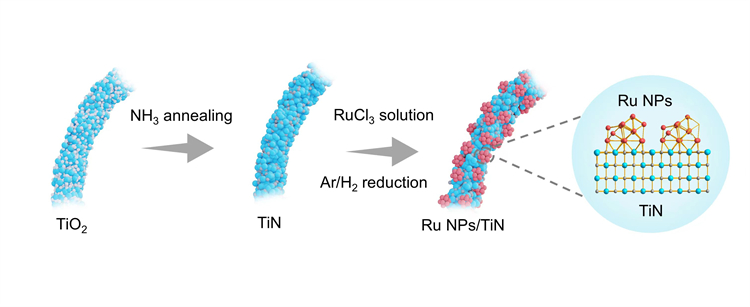
Fig. 1 Synthesis diagram of Ru NPs/TiN
Heterogeneous catalysts composed of supports and precious metals are a key focus in areas such as energy conversion, chemical synthesis, and pollutant degradation. The interaction between supports and precious metals significantly impacts the catalyst’s performance. The research team has discovered that ruthenium nanoparticles exhibit a strong interaction with a titanium nitride support, which effectively regulates the electronic structure of the ruthenium nanoparticles. This interaction optimizes the adsorption energy of hydrogen intermediates, thereby enhancing catalytic activity.
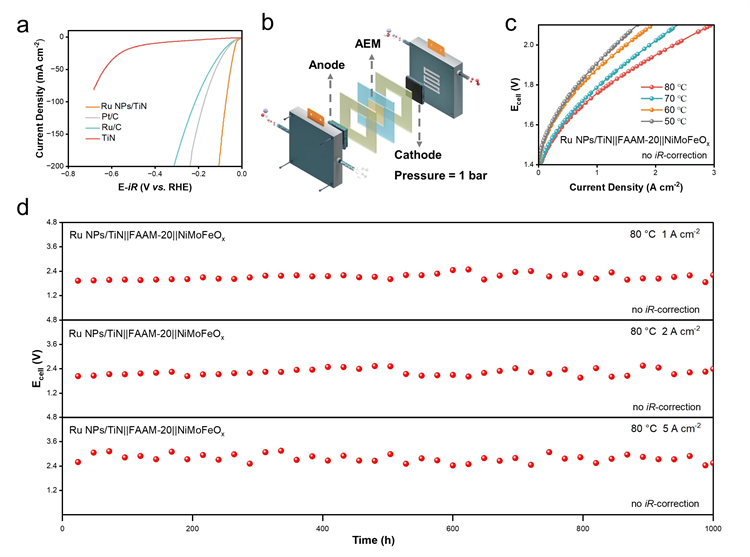
Fig. 2 (a) Performance comparison of Ru NPs/TiN with commercial Pt/C (20%) and Ru/C (5%) in a three-electrode system; Electrolyte: 1 M KOH. (b) Schematic diagram of an AEM electrolyzer. (c) LSV curves of the Ru NPs/TiN||FAAM-20||NiMoFeOx NF AEM electrolyzer at various temperatures. (d) Potential-time curves of Ru NPs/TiN||FAAM-20||NiMoFeOx NF at current densities of 1, 2, and 5 A cm-2. Geometric area of the electrode: 1 cm2; Electrolyte: 30% KOH; Electrolyte temperature: 80 °C.
“We assembled an AEM electrolyzer using Ru NPs/TiN as the HER catalyst, achieving energy efficiencies of 70.1%, 64.3%, and 58.0% at current densities of 0.5, 1, and 2 A cm-2, respectively. The electrolyzer also operated stably for over 1,000 hours at these current densities, with negligible performance degradation,” said Jia Zhao, the first author of the paper and a doctoral student from Nankai University’s College of Electronic Information and Optical Engineering, Class of 2021.
Nankai University is the primary institution and the corresponding institution for this research, with Professor Luo Jingshan of Nankai University and Professor Federico Calle-Vallejo of the University of the Basque Country serving as the corresponding authors.
“At an industrial-level current density of 5 A cm-2, our research outcomes have enabled the AEM electrolyzer to operate efficiently and stably, fulfilling the demands for large-scale commercial application of AEM hydrogen production,” said Professor Jingshan Luo. “Looking ahead, our team remains committed to the independent R&D of green hydrogen production technologies, aiming to accelerate the translation of scientific achievements into practical applications and contribute to the establishment of a green hydrogen energy supply system that is zero-carbon, low-cost, safe and reliable.”
Paper link: https://www.nature.com/articles/s41467-024-50691-5
(Edited and translated by Nankai News Team.)









