NKU Team Breaks New Ground in Developing a Cost-effective Carbon-based Electrocatalyst
A team led by Professor Zhou Qixing from Nankai University, in collaboration with several domestic institutions, has successfully designed and reported a novel and highly efficient noble metal-free bifunctional electrocatalyst for the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR), known as FeOCo-SAD. By adjusting the pyrolysis temperature and the inert atmosphere, they synthesized a catalyst with atomically dispersed oxygen-bridged Fe and Co dual metal dimers active sites (Fe-O-Co-N6).
This work introduces an oxygen bridge that significantly alters the charge distribution around the Fe and Co atoms, thereby greatly enhancing the catalyst’s ability to activate oxygen and fine-tuning the adsorption and desorption of reaction intermediates. This strategic approach has led to a substantial advancement in creating a cost-effective carbon-based electrocatalyst.
The FeOCo-SAD catalyst exhibits superior reversible oxygen catalytic performance for both OER and ORR. Its electrocatalytic capabilities were assessed in an oxygen-saturated 0.1 M KOH alkaline solution by using a standard three-electrode setup, tested at a rotation rate of 1,600 rpm. The FeOCo-SAD sample demonstrated remarkable OER activity, reaching a current density of 10 mA cm-2 with an overpotential of merely 310 mV, which is notably more efficient than the commercial Ir/C (380 mV) catalyst. The derived Tafel slope from the OER polarization curve for the FeOCo-SAD is 80 mV dec-1, significantly lower than that of the Ir/C (90 mV dec-1), suggesting a more rapid OER kinetic process facilitated by the oxygen-bridged Fe and Co dual dimer active sites. After a rigorous 16-hour continuous test, no significant performance degradation was observed for the FeOCo-SAD catalyst, substantiating the structural integrity of the Fe-O-Co-N6 active sites over extended periods. In terms of ORR, FeOCo-SAD also surpasses Pt/C, exhibiting a more favorable onset potential and half-wave potential of 1.07 V and 0.87 V versus the reversible hydrogen electrode (RHE), respectively. Furthermore, the FeOCo-SAD catalyst has shown exceptional long-term stability for ORR, with negligible performance loss even after 30,000 cycles of cyclic voltammetry (CV).
Theoretical computations were conducted to elucidate the relationship between the oxygen-bridged Fe, Co dimeric active sites (Fe−O−Co−N6) and their electrocatalytic capabilities. The Bader charge analysis and differential charge density distribution at the active sites indicated that the incorporation of oxygen atoms to form the bridge resulted in significant charge redistribution around Fe and Co atoms. This redistribution enhances the adsorption and desorption of oxidative intermediates, which in turn affects the catalytic properties. In the oxygen adsorption process, the O−O bond length within the Fe−O−Co−N6 structure is 1.299 Å, which is longer than that of the standard O2 molecule of 1.234 Å. This suggests that the oxygen bridge significantly amplifies the interaction between O2 and the metal sites, promoting the activation of O2. The total density of states (TDOS) analysis revealed that Fe−O−Co−N6 exhibits enhanced electronic conductivity due to an increased number of electronic states crossing the Fermi level. Further computations under pH = 13 conditions determined the ORR free energy diagram for the catalyst. The theoretical limiting potential for the Fe−O−Co−N6 structure was found to be 0.38 V, demonstrating its thermodynamic advantage for catalyzing ORR under alkaline conditions over the comparative models. Additionally, the OER mechanism of the Fe−O−Co−N6 structure under the same pH conditions was simulated. The computations indicated that the oxygen-bridged Fe, and Co dimer can notably lower the energy barrier, thereby substantially enhancing the kinetics of OER. The facilitation of *OH detachment to form OH− and the acceleration of *OOH formation are identified as two critical elementary steps that make FeOCo-SAD an effective bifunctional catalyst for both ORR and OER.
The practical performance of FeOCo-SAD is further assessed in a zinc-air battery. The FeOCo-SAD-based zinc-air battery exhibits an open-circuit voltage (OCV) of 1.52 V, which closely approximates the theoretical voltage of 1.65 V for zinc-air batteries. The FeOCo-SAD-ZAB achieves a remarkable peak power density of 241.24 mW cm−2, which is substantially higher than Pt/C+Ir/C-ZAB (124.46 mW cm−2). Additionally, the FeOCo-SAD-ZAB delivers a specific capacity of 820 mAh g−1 at a current density of 10 mA cm−2, outperforming the Pt/C+Ir/C-ZAB, which offers a specific capacity of 733 mAh g−1. It is noteworthy that the FeOCo-SAD-ZAB shows negligible voltage degradation after 45 hours of continuous operation, highlighting its exceptional long-term endurance.
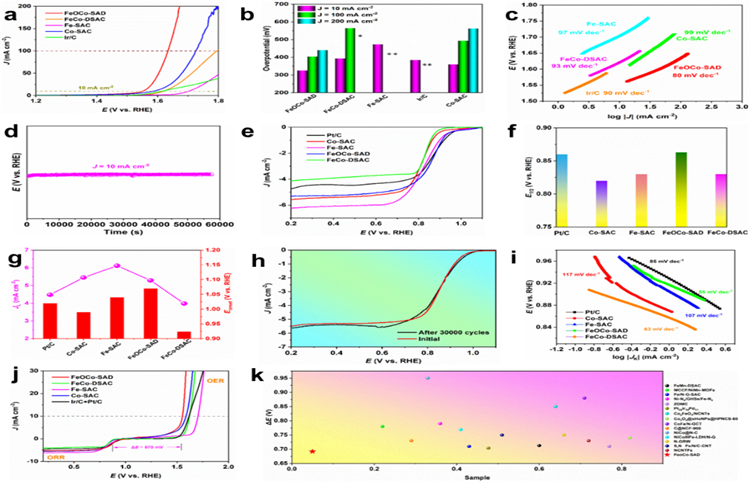
Fig 1 OER performance of FeOCo-SAD
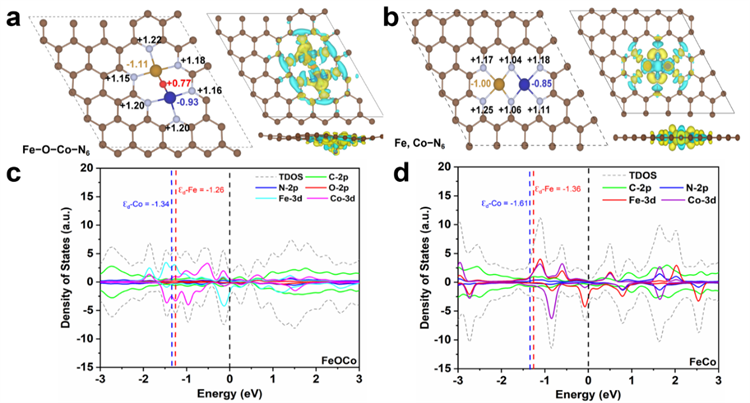
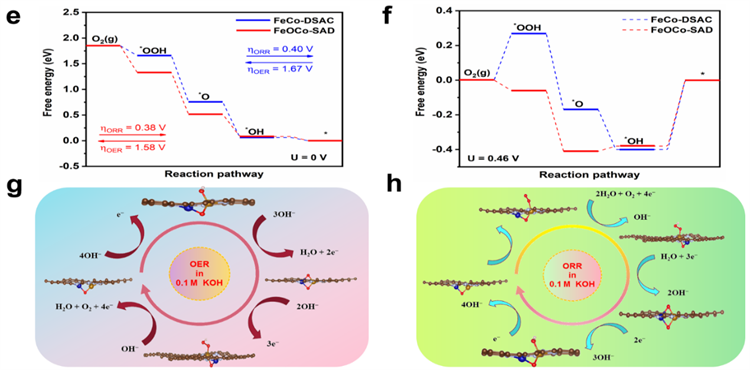
Fig 2 ORR performance of FeOCo-SAD
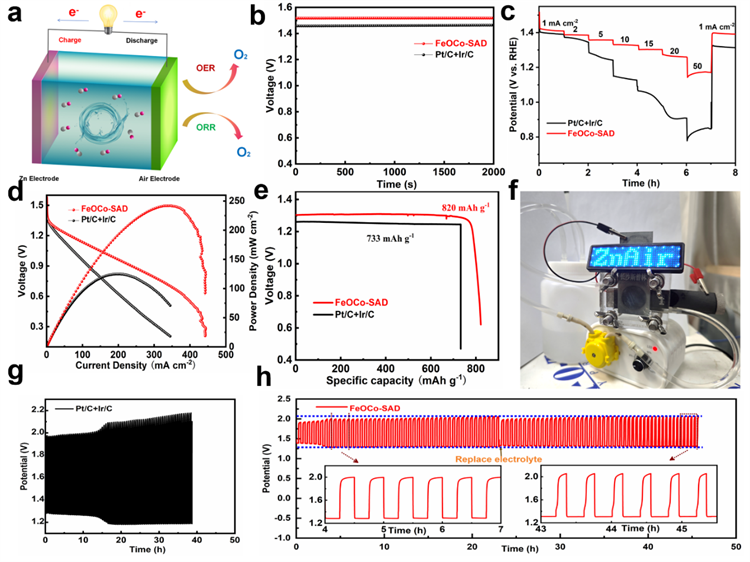
Fig 3 Performance of Zinc-air Battery
The research indicates that the distinctive Fe−O−Co−N6 structure, featuring an oxygen-bridged Fe and Co dual metal dimer, serves as an active center for reversible oxygen electrocatalysis, breaking new ground in the field of non-oxide-based bifunctional electrocatalyst. This breakthrough offers a novel and effective strategy for the design of high-performance ORR/OER catalysts, with potential applications in a variety of sectors, including renewable energy technologies such as zinc-air batteries (ZABs), artificial photosynthesis within the context of the carbon biogeochemical cycle, and the treatment of complex aqueous pollution. Additionally, the research sheds light on the catalytic mechanisms of atomically dispersed catalysts with oxygen-bridge motifs, contributing to a deeper understanding of their behavior in electrocatalytic processes.
This research achievement was published on July 18 with the title “Oxygen Bridging Fe, Co Dual-Metal Dimers Boost Reversible Oxygen Electrocatalysis for Rechargeable Zn–air batteries” in PNAS, a journal that is recognized as one of the most influential in the international scientific community.
Nankai University is the principal and corresponding institution for this research. Professor Zhou Qixing is identified as the first and corresponding author of the publication. Dr. Xue Wendan is the co-first author. The co-corresponding authors are Dr. Wang Pengfei, Dr. Cui Xun of Wuhan Textile University, and Professor Chen Juan of Hohai University.
(Edited and translated by Nankai News Team.)









