NKU Researchers Achieve a Breakthrough in Transition Metal Chemistry
A joint research team led by Prof. Sun Zhongming from Nankai University and Prof. Zhao Lili from Nanjing Tech University has achieved a breakthrough in transition metal chemistry. Their research outcome, titled “Synthesis of Triple-Decker Sandwich Compounds Featuring a M–M Bond Through Cyclo-Bi₅ and Cyclo-Sb₅ Rings”, was recently published in Nature Chemistry. Using Zintl-phase compounds as Sb/Bi sources, the researchers successfully synthesized Cyclo-Bi₅ and Cyclo-Sb₅ rings and achieved the first-ever isolation of Bi₅⁻ anions in a one-step reaction at room temperature. They conducted detailed experimental characterization and theoretical calculations, achieving a significant breakthrough in the field of main-group and transition metal coordination chemistry.
Ligands comprising π-aromatic pentagonal rings have been widely employed in transition metal chemistry. Since the discovery of ferrocene, the cyclopentadienyl anion (C₅H₅⁻), particularly, has been one of the most frequently used ligands. Pn₅⁻, a purely inorganic ligand, can be derived by substituting CR groups in the general formula (C₅R₅)⁻ (where R = H or organic substituents) with isoelectronic Group 15 elements (Pn). Bismuth (Bi), the heaviest pnictogen homolog, has remained elusive and represents a significant synthetic challenge in inorganic synthetic chemistry that persists to date. Lighter pnictogen homologs have been successfully synthesized as such ligands: N₅⁻ can be synthesized via C-N bond cleavage of aryl pentazides with m-CPBA and transition metal complexes serving as stabilizing agents (Nature 2017, 549, 78-81); P₅⁻ and As₅⁻ are typically synthesized through reduction of white phosphorus or yellow arsenic, or alternatively from organic cyclic phosphorus/arsenic compounds. These reactions require elevated temperatures and prolonged reaction times, ultimately yielding stable triple-decker sandwich compounds (Angew. Chem. Int. Ed. 1986, 25, 363-364; J. Am. Chem. Soc. 1982, 104, 4727-4729). The synthesis of Sb₅⁻ and Bi₅⁻ proves more challenging, as these heavy pnictogen atoms preferentially form clusters rather than multiply-bonded rings. To date, only one Sb₅⁻ species has been reported, synthesized from tBu₄Sb₄ as the starting material (Angew. Chem. Int. Ed. 2000, 39, 4148-4150) (Figure 1).
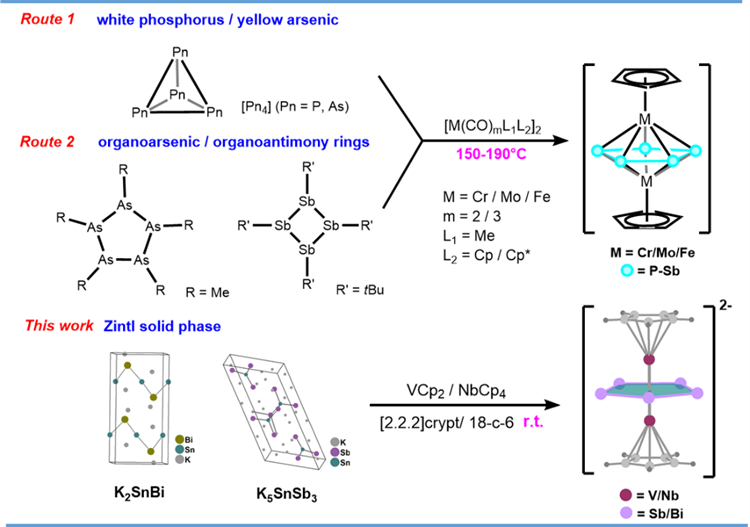
Figure 1. Reported triple-decker sandwich compounds and the synthetic route developed in this work
The research team led by Prof. Sun and Prof. Zhao used Zintl-phase compounds as Sb/Bi sources to synthesize cyclo-Sb₅ and cyclo-Bi₅ and achieve the first-ever isolated Bi5- via a one-step reaction at room temperature. The anionic complexes [CpV(cyclo-Sb₅)V-Cp]²⁻ and [CpNb(cyclo-Bi₅)Nb-Cp]²⁻ feature pentagonal bipyramidal core structures, where the planar Pn₅ rings are sandwiched between two transition metals (V or Nb). The unique V–V and Nb–Nb bonds penetrating the Sb₅/Bi₅ centers exert a significant impact on the electronic structure of these clusters and distinguish them from previously reported neutral sandwich compounds (Figure 2).

Figure 2. The structure and mass spectrum of the cluster [CpNb(cyclo-Bi₅)NbCp]²⁻ (Isostructural to [CpV(cyclo-Sb₅)VCp]²⁻)
Adaptive Natural Density Partitioning (AdNDP) analysis of [V₂Cp₂Sb₅]²⁻ reveals only two 3c-2e bonds in the Sb₅ ring, in addition to ten 2c-2e V-Sb bonds, five 1c-2e lone pairs, as well as a V-V bond with high occupancy up to 1.93. AdNDP analysis of [Nb₂Cp₂Bi₅]²⁻ reveals bonding patterns analogous to its [V₂Cp₂Sb₅]²⁻ counterpart (Figure 3).

Figure 3. QTAIM and AdNDP analyses of [V₂Cp₂Sb₅]²⁻ and [Nb₂Cp₂Bi₅]²⁻
To verify the existence of a 2c-2e M–M bond penetrating the E₅ ring center in the M(cyclo-E₅)M core unit, the research team conducted EDA-NOCV calculations on [V₂Cp₂Sb₅]²⁻ and [Nb₂Cp₂Bi₅]²⁻. They used cyclo-Sb₅/Bi₅ and (VCp)₂/(NbCp)₂ as interacting fragments and conducted analyses under varying charge and electronic states. Analysis of the deformation densities and bonding orbitals reveals that ten orbital interactions originate from M-E₅ cage bond formation, while one distinct orbital interaction is unequivocally associated with M-M bonding, with negligible contribution (ΔEₒᵣb(4) from the E₅⁻ ring (∆Eₒᵣb(4) in [V₂Cp₂Sb₅]²⁻ and ∆Eₒᵣb(1) in [Nb₂Cp₂Bi₅]²⁻), which nevertheless represents the strongest orbital contribution in these molecules. The Nb–Nb bond formed through the orbital interaction ΔEorb(1) primarily involves the HOMO-3 and LUMO of the [(NbCp)₂]⁻ fragment. The corresponding charge flow eigenvalue (0.074) confirms negligible contribution from the [cyclo-Bi5]- unit. This phenomenon is mirrored in the ∆Eorb(4) interaction of [V2Cp2Sb5]2- (Figure 4), conclusively demonstrating that both V–V and Nb–Nb bonds physically penetrate the central cavity of the E5 rings.

Figure 4. The deformation density (Δρ) and corresponding interfragment orbital interaction associated with the dominant ∆Eorb(1) term in [Nb2Cp2Bi5]2-
Dr. Xu Yuhe from NKU and Yang Xing from NTU equally as co-first authors. Corresponding authors include Prof. Sun Zhongming from NKU, Prof. Zhao Lili from NTU, and Prof. Gernot Frenking from Philipps-Universität Marburg, Germany (Adjunct Professor at NTU).
Read the paper at https://www.nature.com/articles/s41557-025-01765-4
(Edited and translated by Nankai News Team.)









