A Research Team from Nankai University Reports Key Discovery in Acid Zero-Gap Membrane Electrode Assembly Carbon Dioxide Electrolyzers
Recently, Prof. Luo Jingshan’s research group at the College of Electronic Information and Optical Engineering, Nankai University (NKU) reported new findings in the field of acidic membrane electrode assembly for electrocatalytic carbon dioxide (CO₂) reduction in Nature Communications. The research team proposed using porous membrane in place of conventional Nafion membrane to enable highly selective and stable electrocatalytic CO₂ conversion under acidic conditions, thereby providing a new solution for carbon dioxide resource utilization.
CO₂ is the predominant greenhouse gas today. Transforming CO₂ from a waste product into a valuable resource is crucial for achieving carbon peaking and carbon neutrality. For high energy efficiency and highly integrated functionality, zero-gap membrane electrode assembly carbon dioxide electrolyzers have long been considered a promising direction for CO₂ conversion. However, they can hardly maintain both stability and high carbon utilization due to frequent occurrence of salt precipitation, flooding, the competing hydrogen evolution reaction, and other operational challenges.
The research team proposed replacing the core “transportation component” to resolve the above issues. They substituted conventional Nafion membrane with porous membrane to construct zero-gap membrane electrode assembly electrolyzer under acidic conditions. The use of porous membrane with appropriate pore size and moderate hydrophilicity enabled balanced mass transport, thereby fundamentally improving the reaction environment. This design effectively suppressed salt precipitation and the hydrogen evolution reaction, allowing the system to maintain high performance even at high current densities.
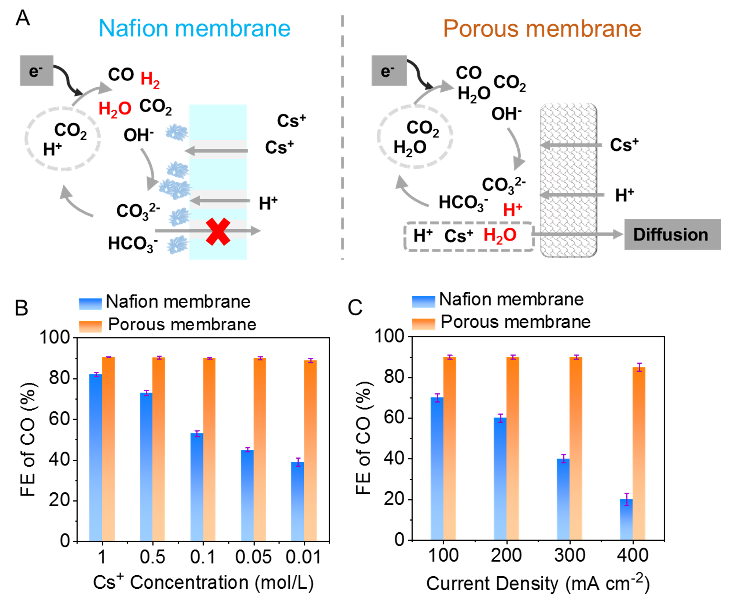
Fig. 1 (A) Transport characteristics of Nafion membrane (left) versus porous membrane (right) under an electric field. (B) Comparative tests of CO Faradaic efficiency under different ion concentrations. (C) Comparative tests of CO Faradaic efficiency at different current densities.
Experimental results demonstrated that in an acidic electrolyte, the porous membrane electrolyzer achieved a CO Faradaic efficiency of up to 85% at a current density of 400 mA cm⁻². In contrast, the Nafion membrane showed less than 20% CO Faradaic efficiency under identical conditions. The performance gap clearly revealed the significant superiority of the porous membrane design. Furthermore, during a 200-hour continuous operation for a long-term stability test, the CO selectivity approached 100%, with minimal hydrogen evolution and no observable salt precipitation. In a scaled-up 100 cm² electrolyzer test, the system maintained stable operation for over 120 hours with CO selectivity remaining around 90%. These experimental results fully validated the design’s scalability and potential for industrial application.
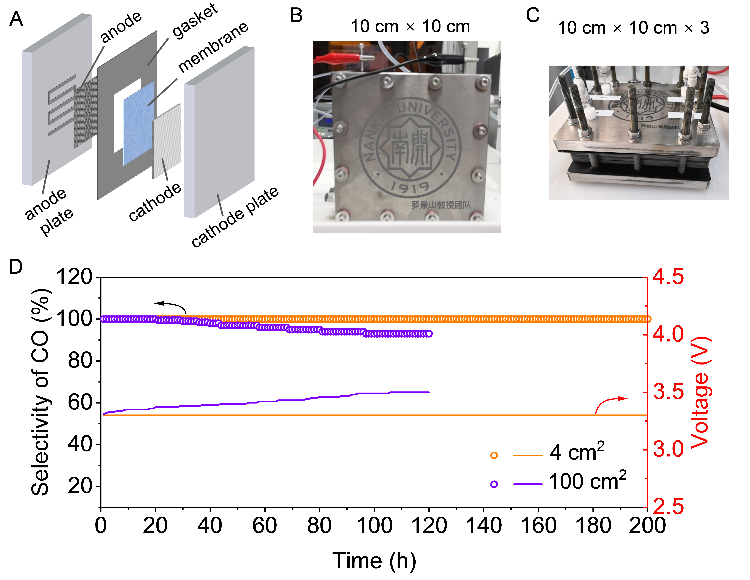
Fig. 2 (A) Components of a membrane electrode assembly electrolyzer. (B) Schematic of a 100 cm² electrolyzer cell. (C) Schematic of a 300 cm² electrolyzer stack (3-cell assembly). (D) Long-term stability test.
“This finding provides a novel membrane utilization and design strategy for acidic membrane electrode assembly CO₂ reduction electrolyzers, thus laying the groundwork for the future electrosynthesis of high-value-added carbon-based fuels and chemicals,” stated Prof. Luo Jingshan. He emphasized his research group’s ongoing commitment to addressing scientific challenges in CO₂ conversion. He also outlined the team’s planned focus on the optimization of electrode interface design and the translation of laboratory CO₂ electrolysis technology toward industrial implementation, thereby contributing NKU’s expertise to the development of new quality productive forces.
NKU served as both the sole research institution and the corresponding affiliation for this study. Prof. Luo Jingshan is the corresponding author and Wei Shilei the first author of the paper.
Read the paper at https://www.nature.com/articles/s41467-025-64342-w.
(Edited and translated by Nankai News Team.)









