Nankai University Team Achieves Major Breakthrough in Stem Cell Totipotency Activation
A research team led by Professor Shuai Ling at the State Key Laboratory of Chemical Medical Biology at Nankai University, has discovered that knocking out the Sorcs3 gene in mouse embryonic stem cells (ESCs) efficiently activates totipotent phenotypes. The underlying mechanism appears to be closely associated with activation of the core transcription factor Tfap2c. Further investigation revealed that acquisition of these totipotent features is primarily achieved through inhibition of three critical signaling pathways, offering new strategies for developing efficient and reliable totipotency culture systems. The findings were published in Advanced Science.
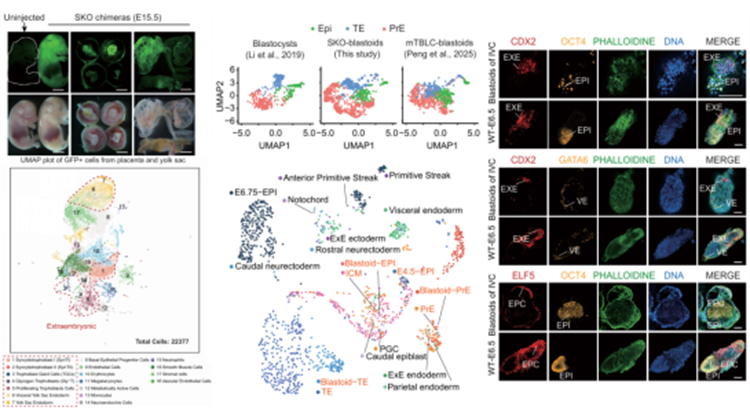
In this study, the team successfully established Sorcs3 knockout (SKO)-ESCs (SKO-ESCs), which acquired the ability to differentiate into TSC-like cells and XEN-like cells. Chimera formation assays and single-cell RNA sequencing demonstrated that SKO-ESCs readily contribute not only to the embryo but also to extraembryonic tissues such as the placenta and yolk sac. Knockout of the downstream key gene Tfap2c severely impaired this capacity. Moreover, in 3D in vitro culture systems, SKO-ESCs efficiently self-assembled into human blastocyst-like structures (blastoids) that closely resembled wild-type blastocysts in structure, cellular composition, and transcriptomic features. These blastoids could develop to a stage comparable to embryonic day 6.5 (E6.5) in vitro and expressed key post-implantation lineage markers such as ELF5, providing an ideal in vitro model for studying early embryonic development.
Further analysis of Tfap2c-positive cells in SKO-ESCs revealed that Sorcs3 knockout suppresses three key signaling pathways: TGF-β, PI3K-AKT, and lysosome. By adding a combination of inhibitors targeting these three pathways—SB-431542, LY294002, and Vacuolin-1 (collectively termed SLV)—to wild-type ESC culture medium, the team successfully induced totipotency without genetic manipulation. SLV-treated ESCs not only efficiently formed blastoids but also contributed robustly to both embryonic and extraembryonic tissues in chimeric assays. These cells maintained karyotypic stability and normal cellular viability during long-term culture, with global DNA methylation levels reduced to 32%, closely resembling those of natural totipotent cells.
This breakthrough not only reveals, for the first time, the novel role of Sorcs3 as a “restriction factor” for totipotency, but also establishes two efficient and stable strategies for obtaining totipotent stem cells: knockout of Sorcs3 and small-molecule-based inhibition of three signaling pathways. The former provides an ideal model for dissecting the molecular network regulating totipotency, while the latter—being operationally simple and highly safe—lays a foundation for establishing standardized totipotent stem cell lines in regenerative medicine.
The Sorcs3 gene plays a critical role in activating stem cell totipotency
Professor Shuai Ling commented, “The high-quality blastoid model developed in this study addresses the challenge of limited early embryonic material availability, providing a reliable tool for investigating mammalian implantation mechanisms, cell fate determination, and developmental disorders. This achievement represents not only a major breakthrough in developmental biology but also advances stem cell engineering and regenerative medicine a critical step toward simulating complete embryonic development and achieving functional tissue regeneration.”
Associate Professor Zhang Wenhao and doctoral student Mao Xinyu from Nankai University, along with Associate Professor He Yu from the Children’s Hospital of Chongqing Medical University, are co-first authors of the paper. Professor Shuai Ling, Dr. Gao Qian from the State Key Laboratory of Chemical Medical Biology at Nankai University, and Professor Shi Yuan from the Children’s Hospital of Chongqing Medical University, served as co-corresponding authors.
Link to the paper: https://doi.org/10.1002/advs.202509151
(Edited and translated by Nankai News Team.)









