Nankai University-Tsinghua University Joint Research Team Reports Key Discovery in Cell
The Nankai University-Tsinghua University Structure-Based Drug Discovery Team—a joint research team comprising Research Fellow Huang Yucen and Academician Rao Zihe from the State Key Laboratory of Medicinal Chemical Biology, Nankai University (NKU), alongside Prof. Lou Zhiyong from Tsinghua University—in collaboration with Prof. Zhu Lan’s team from Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, and other institutions, has uncovered a concurrence mechanism of RNA template recycling and RNA capping during coronavirus transcription and replication, which has redefined the virology field’s understanding of RNA virus transcription and replication. The related findings were published online in the journal Cell on October 22, marking another significant achievement by the joint research team in their study of coronavirus transcription and replication mechanisms, following a series of high-impact publications in journals such as Science and Cell since 2020.

With a focus on the coronavirus transcription and replication process over the past five years, the research team has successively elucidated the working mechanisms of C-RTC, E-RTC, Capping Intermediate Complex, and Cap0 Capping Complex. Building on this foundational work, they designed a series of novel nucleic acid scaffolds and successfully captured two super-complexes—the Pre-Capping Initiation (Pre-CI) and Post-Capping Initiation (Post-CI) states of the transcription-replication complex. This finding elucidated the molecular mechanism by which RNA template recycling and RNA capping initiation occur synergistically (see Fig. 1).
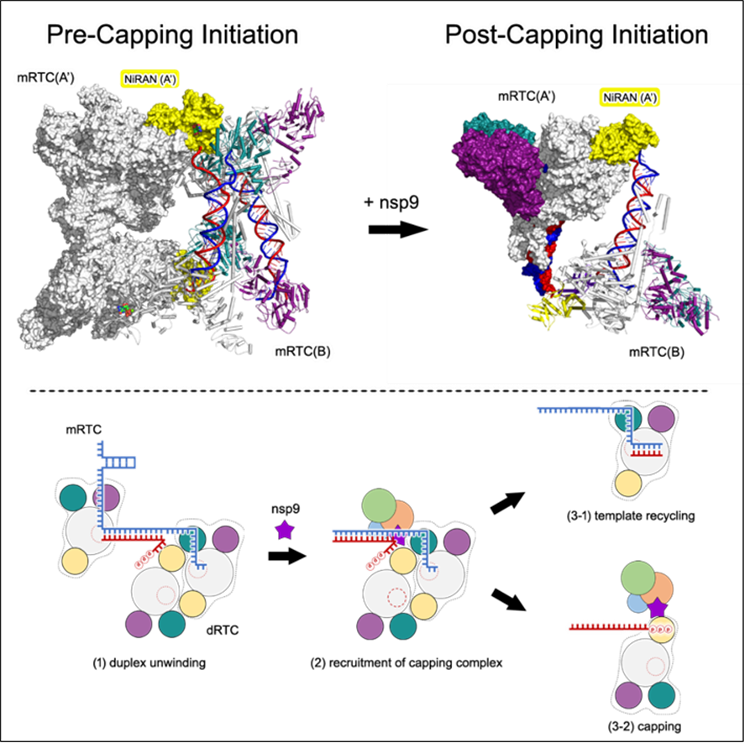
Fig. 1. Coronavirus template recycling and RNA capping initiation concurrence mechanism model
The research team designed a double-stranded RNA scaffold and utilized the highly reactive SARS-CoV-2 helicase along with other transcription-replication enzymes to assemble the Pre-CI complex (Fig. 2). This complex constitutes a super-complex in a tetrameric form, composed of four E-RTCs. Within the super-complex, the “template-product” double-stranded RNA emanating from the catalytic site of polymerase nsp12 is unwound into single strands by a helicase from another elongation complex. The resulting single-stranded RNA then serves as a new template for RNA synthesis, thereby enabling the recycling of the template RNA.
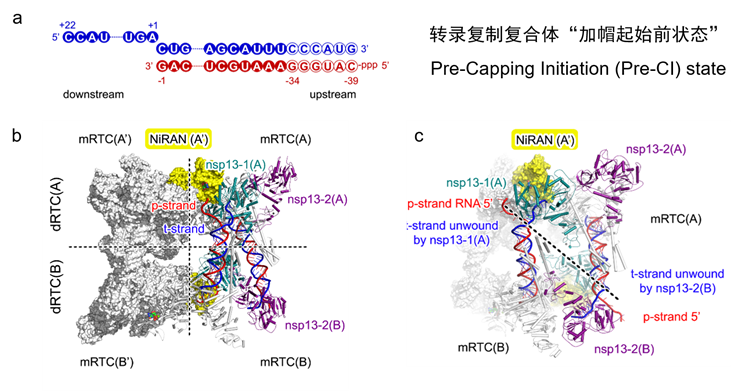
Fig. 2. (a) The RNA scaffold used for assembling the Pre-CI complex. (b) Overall architecture of the Pre-CI complex in its tetrameric form. (c) Unwinding of the “template-product” double-stranded RNA within the Pre-CI complex.
Another pivotal discovery within the Pre-CI complex was the team’s first definitive identification of the coronavirus helicase’s 3’-5’ unwinding polarity. The study further pinpointed critical structural elements responsible for unwinding and visualized the dynamic process of double-stranded RNA unwinding driven by ATP hydrolysis (see Fig. 3). Since the initial identification of the coronavirus helicase in 2000, the unwinding polarity had been presumed to be 5’-3’, which, however, conflicted with multiple established observations and remained a long-standing controversy. Employing structural biology approaches, the study has now directly and unambiguously demonstrated the 3’-5’ unwinding direction of the coronavirus helicase, thereby resolving this enduring debate.
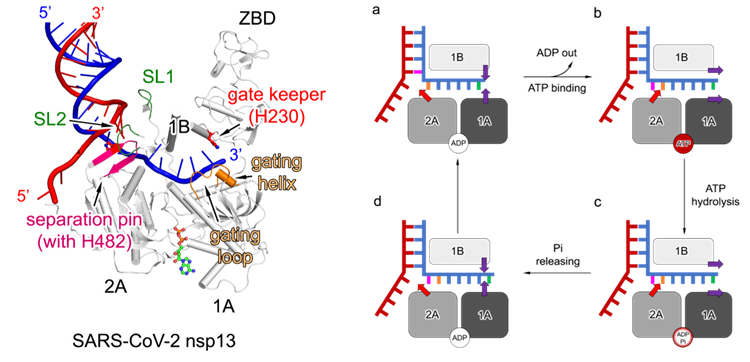
Fig. 3. Left: Key structural elements of the SARS-CoV-2 helicase. Right: Schematic model of double-stranded RNA unwinding driven by ATP hydrolysis.
Subsequently, the research team designed and synthesized a double-stranded RNA scaffold with a single-stranded overhang. They introduced the nsp9 protein into the system to successfully capture the Post-CI complex (Fig. 4). With the presence of nsp9, the complex underwent a structural transition from a tetrameric form during the Pre-CI state to a dimeric form during the Post-CI state. Most critically, the triphosphate group at the 5’ end of the RNA product strand and the N-terminal amino acid of the nsp9 protein were found to co-localize within the catalytic site of the polymerase’s NiRAN domain. This finding clearly demonstrates that the 5’ end of the nascent RNA product strand directly enters the nsp12 NiRAN domain and initiates the RNA capping process via an nsp9-mediated RNAylation mechanism. Thus, this study has not only transformed the understanding of the coronavirus capping process but also provided clear structural biological evidence supporting the research team’s previously proposed NiRAN-catalyzed, GTP-dependent RNA capping mechanism.
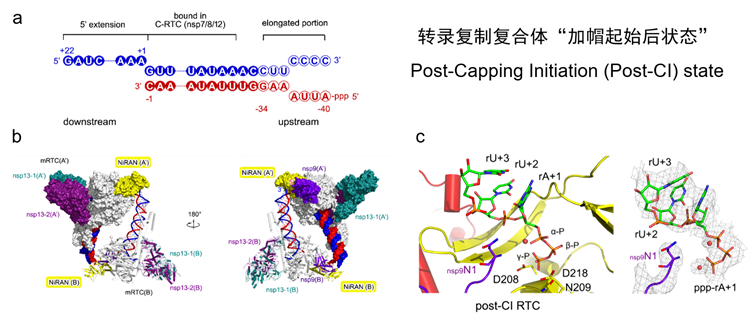
Fig. 4. (a) RNA scaffold used for the assembly of the Post-CI complex. (b) Overall architecture of the Post-CI complex. (c) Co-localization of the RNA product strand 5’-triphosphate group with the nsp9 protein at the catalytic center of the NiRAN domain within the Post-CI complex
This finding has fundamentally reshaped the understanding of the viral transcription-replication process and settled a 25-year-long debate over the polarity of the coronavirus helicase and its RNA capping mechanism. This breakthrough culminates from two decades of sustained research in the field of coronavirus transcription and replication. Since 2020, the research team has systematically studied the SARS-CoV-2 transcription-replication process, and elucidated the three-dimensional structures of multiple states of the transcription-replication complex, which has provided crucial information for understanding the viral life cycle and developing potent antiviral therapeutics. Furthermore, the research team has conducted in-depth studies on SARS-CoV-2 infection of the female reproductive tract and the impact of inactivated COVID-19 vaccination on maternal-fetal health. Their findings, published as a series of high-impact papers in top-tier international journals such as Science, Cell, and Nature Communications, represent one of the most systematic and frequently cited research efforts on SARS-CoV-2 transcription and replication globally. This study has been recognized through numerous prestigious honors, including China’s Top 10 Scientific Advances, Top 10 Advances in Life Sciences, Key Medical Advances of 2021, and Chinese Medical Science and Technology Award.
Dr. Yan Liming from Tsinghua University and Research Fellow Huang Yucen from the NKU State Key Laboratory of Medicinal Chemical Biology served as co-first authors of the paper. Prof. Lou Zhiyong from Tsinghua University, Academician Rao Zihe from NKU, and Prof. Zhu Lan from Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, were the corresponding authors.
Read the paper at https://www.cell.com/cell/abstract/S0092-8674(25)01090-6.
(Edited and translated by Nankai News Team.)









